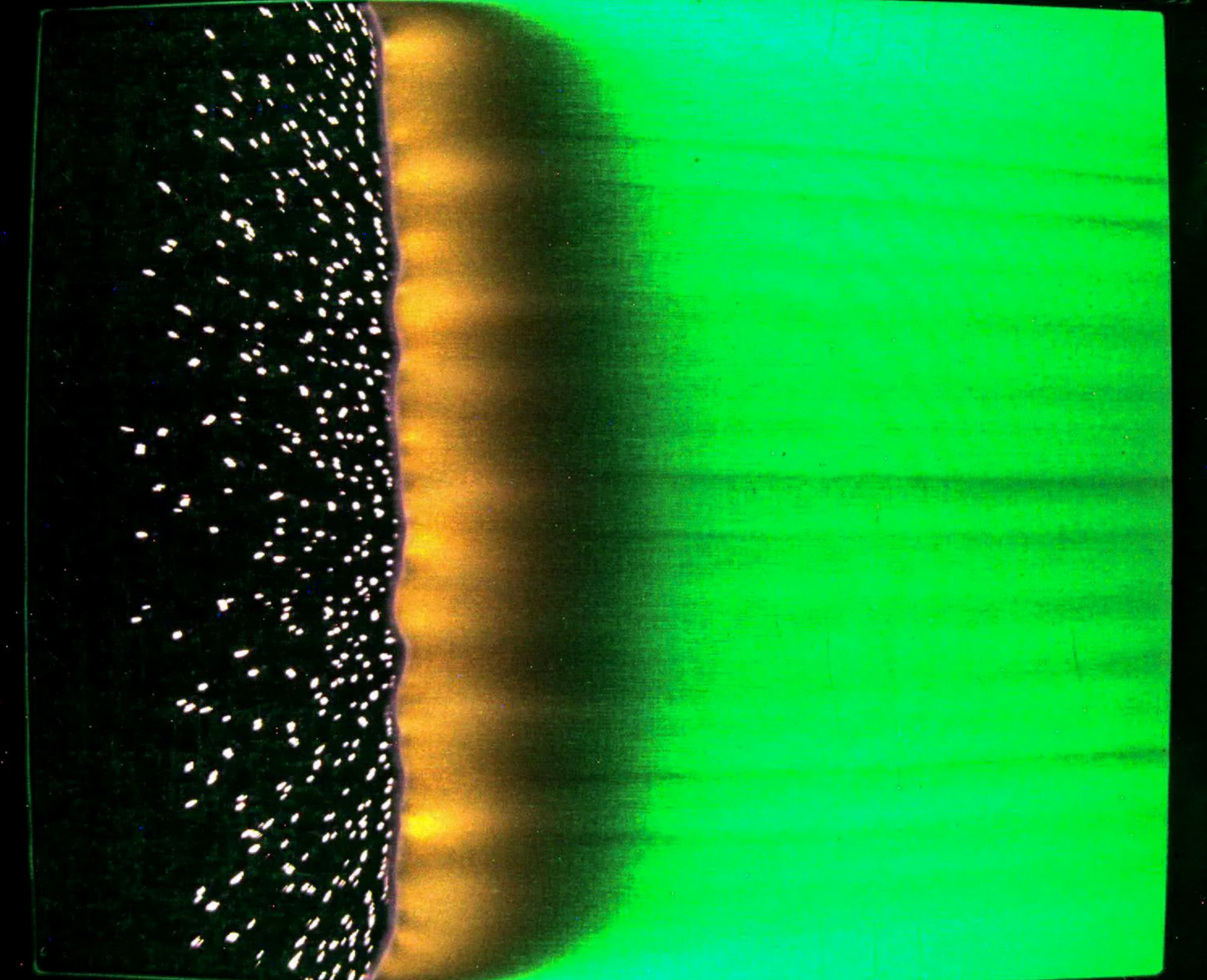
Astronaut Don Pettit posted this image of a Jupiter-like water globe he created on the International Space Station. In microgravity, surface tension reigns as the water’s supreme force, pulling the mixture of water and food coloring into a perfect sphere. It will be interesting to see a video version of this experiment, so that we can tell what tools Pettit used to swirl the droplet into the eddies we see. Is the full droplet rotating (as a planet would), or are we just seeing the remains of a wire passed through the drop? We’ll have to stay tuned to Pettit’s experiments to find out. (Image credit: NASA/D. Pettit; via space.com; submitted by J. Shoer)
Tag: microgravity

Ice Without Gravity
Astronaut Don Pettit is back in space, and that means lots of awesome microgravity experiments. Here, he grew thin wafers of ice in microgravity in a -95 degree Celsius freezer. Then he took the ice wafers and photographed them between crossed polarizers, creating this colorful image. The colors highlight different crystal orientations within the ice and give us a hint about how the freezing front formed and expanded. I can’t wait to see more examples! (Image credit: D. Pettit/NASA; via Ars Technica; submitted by J. Shoer)

Farewell, Saffire!
After eight years and six flight tests, NASA said a fiery farewell to the Spacecraft Fire Safety Experiment, or Saffire, mission. Each Saffire test took place on an uncrewed Cygnus supply vehicle after undocking from the space station. Cygnus craft burn up during atmospheric re-entry, so using them as a platform guaranteed safety for the station’s crew.
A Plexiglass sample burns as part of Saffire-V’s experiments. In this experiment, researchers found that flames grew and spread faster on thin ribs of Plexiglass (left) than on thicker samples (right). Saffire itself used a small wind tunnel to push air past its burning materials. The tests included materials like plexiglass, cotton, Nomex, and other fabrics that might be found on a spacecraft or its occupants. The goal, of course, is to understand how fires grow and spread in a spacecraft in order to protect the crew. To that end, Saffire experiments recorded not only what went on inside their test unit, but also what the conditions were in the spacecraft as Saffire burned. (Image and video credit: NASA; via Gizmodo and NASA Glenn)

Droplet Medusa
Vibration is one method for breaking a drop into smaller droplets, a process known as atomization. Here, researchers simulate this break-up process for a drop in microgravity. Waves crisscrossing the surface create localized craters and jets, making the drop resemble the Greek mythological figure of Medusa. With enough vibrational amplitude, the jets stretch to point of breaking, releasing daughter droplets. (Image and research credit: D. Panda et al.)

Free Contact Lines
How a simple drop of water sits on a surface is a strangely complicated question. The answer depends on the droplet’s size, its chemistry, the roughness of the surface, and what kind of material it’s sitting on. Vetting the mathematical models that describe these behaviors is especially difficult since droplets often get stuck, or “pinned,” along their contact line where water, air, and surface meet.
To get around this issue, researchers sent their experiment to the International Space Station, asking astronauts to run the tests for them. Without gravity‘s influence squishing drops, the astronauts could use much larger droplets than they could on Earth. Larger drops are less likely to get pinned by a stray surface defect, so on the space station, astronauts could place droplets on a vibrating platform and observe their contact line freely moving as the drop changed shape. Under these conditions, the experiment tested many surfaces with different wetting characteristics, thereby gathering data to test models we cannot easily confirm on Earth. (Image and research credit: J. McCraney et al.; via APS Physics)

Cleaning Up Combustion
In space, flames behave quite differently than we’re used to on Earth. Without gravity, flames are spherical; there are no hot gases rising to create a teardrop-shaped, flickering flame. In many ways, removing gravity makes combustion simpler to study and allows scientists to focus on fundamental behaviors. It’s no surprise, then, that combustion experiments are a long-standing feature on the International Space Station.
In the photo above, we see a flame in microgravity studded with bright yellow spots of soot. Soot is a by-product of incomplete combustion; it’s essentially unburned leftovers from the chemical reaction between fuel and oxygen. In this experiment, researchers were studying how much soot is produced under different burning conditions, work that will help design flames that burn more cleanly in the future. (Image and video credit: NASA; submitted by @LordDewi)

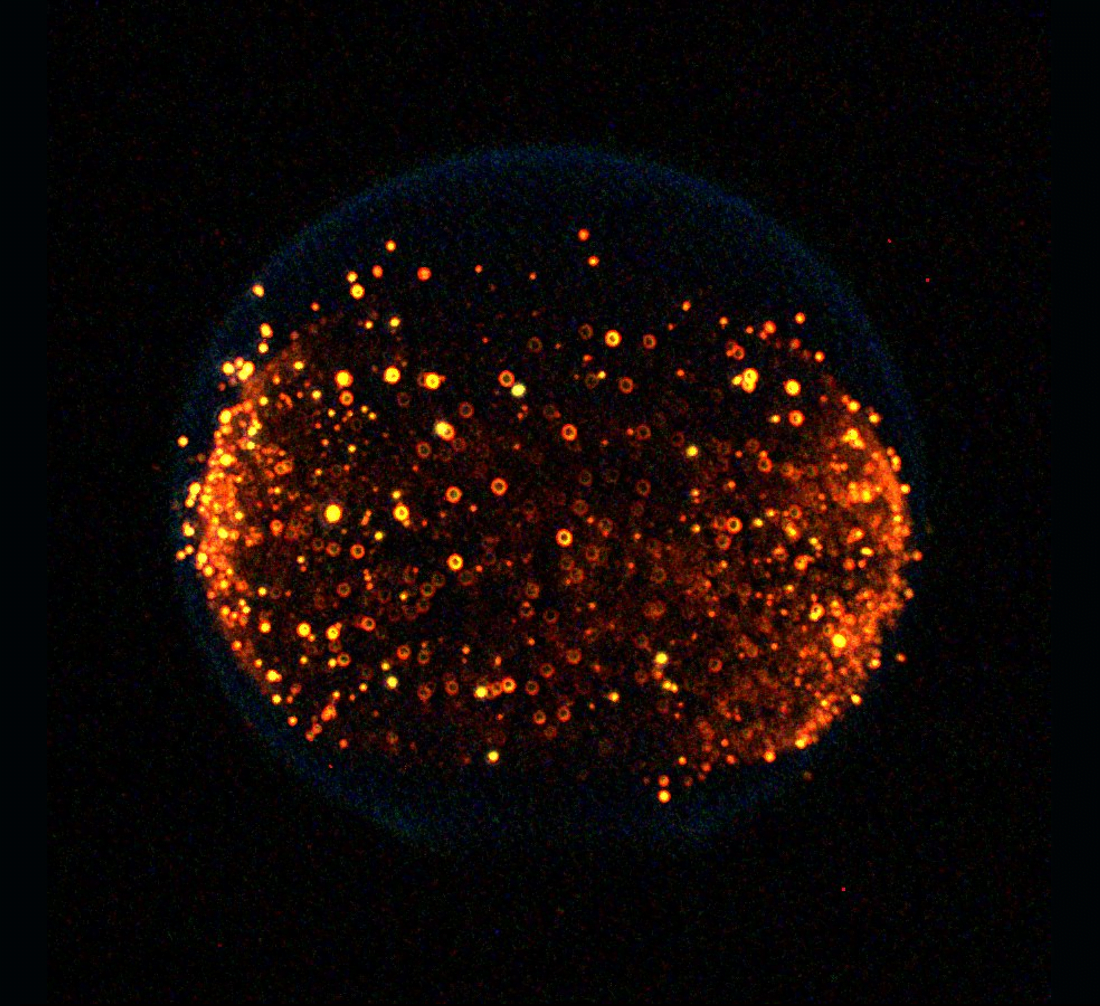
Testing Granular Gas Theory
When excited, a group of particles can behave much like a gas. These granular gases exhibit many similarities to molecular gases but contain one vital difference: without a constant input of energy, granular gases lose kinetic energy to collisions.
Over the years, scientists have developed a special theory to describe the behaviors of granular gases, but most of its predictions could only be tested numerically. A new study used a microgravity experiment aboard a sounding rocket to physically test the theory.
The experiment, shown above, consists of nearly 2800 magnetic particles, which the researchers could stir up using pairs of magnets. Once they shut off the magnets (which occurs at t=0 in the image above), the granular gas begins to “cool” as collisions sap away its energy. With this set-up, the researchers were able to confirm several key predictions of the granular gas theory. (Image and research credit: P. Yu et al.; via APS Physics)

Boiling in Microgravity
In the playground of microgravity, every day processes can behave much differently. This photo comes from the RUBI experiment, the Reference mUltiscale Boiling Investigation, aboard the International Space Station. Freshly installed and switched on, the apparatus is now generating bubbles like this one. On the left, you see temperature sensors used to measure bubble temperatures. High-speed and infrared cameras are also part of the experiment.
The advantage of studying boiling in space is a lack of gravity that can mask or overwhelm subtler effects. It effectively slows down the process, making it easier to observe. And since boiling is such an important part of heat transfer in many manmade devices, it shows us how we have to adapt when operating in an environment where heat – and bubbles – don’t automatically rise. (Image credit: ESA; submitted by Kam-Yung Soh)

Drinking Coffee in Space
You probably don’t give much thought to the forces involved in drinking here on Earth. That’s because gravity’s effects dominate over everything else. Our cups are designed to hold a liquid until we use gravity to pour it into our mouths. But that technique doesn’t work in microgravity. There other forces govern how liquids flow: specifically surface tension and capillary action.
Both of these forces are the result of intermolecular attractions. In the case of surface tension, it’s the attraction that the molecules of a liquid feel for one another that keeps them in a cohesive bunch. Capillary action is similar, but it’s an attraction between the liquid molecules and those of the solid they’re wetting. When you combine them both, you get the ability for liquids to climb up a narrow gap and pull more liquid up behind them. That’s the key science behind every version of the “space cup” developed by astronaut Don Pettit and his collaborators.
To hear more about the development and engineering of the cup (and exactly why it makes drinking coffee so much more enjoyable in space than it would be otherwise) check out the full video. And, in case you’re wondering, there’s a special microgravity champagne flute, too! (Image and video credit: It’s Okay to Be Smart)

Forming Asteroids
Amidst the swirling gas and dust surrounding young stars, asteroids and planets form. Just how these bodies come together – especially before they are massive enough to exert any significant gravitational potential – is an open question. Researchers are trying to better understand the physics involved by studying how clusters of granular material behave when impacted.
Above you see footage from two experiments. Both take place in a drop tower under vacuum conditions. That means the effects of air drag and gravity are removed, just like in space. On the left, the cluster is made up of soft clumps of dust; on the right, the cluster contains hard glass beads. Surprisingly, the researchers found that the two different materials behave the same way. They were able to describe both sets of impacts with exactly the same model. This suggests there may be an underlying universal behavior behind all of these granular materials, though the researchers note more experiments are needed. (Image and research credit: H. Karsuragi and J. Blum; via APS Physics)