When supercooled, water can remain a liquid even below its freezing point. As explained in this Minute Physics video, this happens because of a tug-of-war between effects in the water. Generally speaking, having impurities in the water or smacking the bottle will shift that battle enough for freezing to win out. But it’s possible–theoretically, at least–to create a situation where supercooled water can never freeze. (Video and image credit: Minute Physics)
Tag: supercooling

Hole Punch Clouds
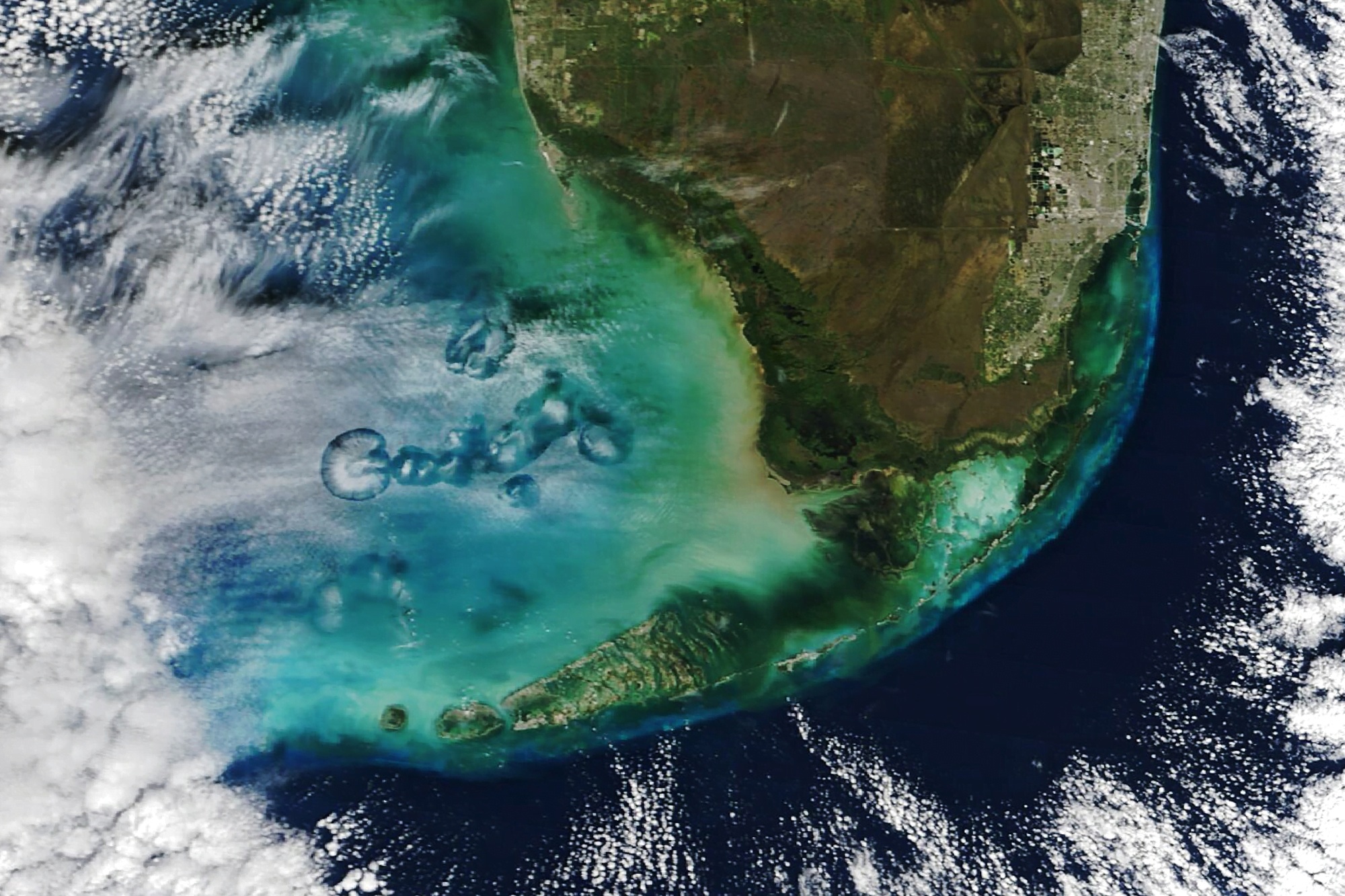
At times altocumulus cloud cover is pierced by circular or elongated holes, filled only with the wispiest of virga. These odd holes are known by many names: cavum, fallstreak holes, and hole punch clouds. Long-running debates about these clouds’ origins were put to rest some 14 years ago, after scientists showed they were triggered by airplanes passing through layers of supercooled droplets.
When supercooled, water droplets hang in the air without freezing, even though they are colder than the freezing point. This typically happens when the water is too pure to provide the specks of dust or biomass needed to form the nucleus of an ice crystal. But when an airplane passes through, the air accelerated over its wings gets even colder, dropping the temperature another 20 degrees Celsius. That is cold enough that, even without a nucleus, water drops will freeze. More and more ice crystals will form, until they grow heavy enough to fall, leaving behind a clear hole or wisps of falling precipitation.
In the satellite image above, flights moving in and out of Miami International Airport have left a variety of holes in the cloud cover each of them large enough to see from space! (Image credit: M. Garrison; research credit: A. Heymsfield et al. 2010 and A. Heymsfield et al. 2011; via NASA Earth Observatory)

Fallstreak Holes
Occasionally clouds appear to have a hole in them; these are known as fallstreak holes or hole-punch clouds. To form, the water droplets in the cloud must be supercooled; in other words, they must be colder than their freezing point but still in liquid form. When disturbed — say, by the temperature drop caused by flowing over an airplane wing — the supercooled water droplets will suddenly freeze. This typically kicks off a chain reaction in which many droplets freeze and the heavy ice crystals fall out of the sky, leaving behind a void in the cloud. Because airplanes are particularly good at creating these fallstreak holes, they’re often seen near busy airports. (Image credit: J. Stevens/NASA; via NASA Earth Observatory)

Dual Structure of Water
Water is so ubiquitous in our lives that we rarely recognize just how strange it is. For example, when pure liquid water is supercooled well below its freezing temperature, it takes on not one but two molecular arrangements, one of which is high-density and one of which is low-density. Theory had posited this configuration for some time, but only recently has experimental evidence supported it.
The experimental challenge was water’s rapid crystallization in the temperature region of interest. Any time water was held at those temperatures in order to study it, it would crystallize before researchers could make their observations. To get around this, a team studied extremely thin layers of water which they heated with a laser before rapidly cooling. By repeating this heating-and-cooling cycle many times, they were able to measure water properties that only make sense if it conforms to the two-density theory. (Image credit: T. Holland/Pacific Northwest National Laboratory; research credit: L. Kringle et al.; via Science News; submitted by Kam-Yung Soh)

Freezing Drop Impact
At the altitudes where aircraft fly, it’s often cold enough for water drops to freeze in seconds or less. Once attached to a wing, such frozen drops disrupt the flow, reducing lift and increasing drag. To help understand how such droplets freeze, scientists study droplet impact on cold surfaces. Starting at room temperature (counter-clockwise from upper left), a drop will spread on the surface, then retract. When the temperature is colder, parts of the droplet freeze before retraction completes, leaving a thin sheet with a thicker center. At even colder temperatures, the droplet’s rim destabilizes and freezing occurs before the droplet has time to retract fully. And at the coldest temperatures, the droplet breaks apart into a frozen splash. (Image and video credits: V. Thievenaz et al.)

“Ice Formations”
As perfect as ice can appear, it always starts with a defect. Without a speck of dust or soot to act as a seed, supercooled water simply will not freeze. But these imperfections can lead to beauty. In “Ice Formations,” photographer Ryota Kajita captures some of the oddities of ice in Alaska’s interior swamps and ponds. In Kajita’s images bubbles are frozen in suspension, plates of ice form strange shapes, and star-shaped cracks peek through the snow. Whether the ice formed too quickly or too slowly, there are interesting signatures left behind. See the full set of images, spanning the last eight years, here. (Image credit: R. Kajita; via Colossal)

Frost Spreading
Frost typically forms when supercooled droplets of water scattered across a surface freeze together. The freezing spreads via tiny ice bridges that link droplets together into a frozen network. The animation above shows this process in action. Freezing starts in a droplet off-screen on the right and quickly spreads. Watch carefully, and you can see the ice bridges growing toward the unfrozen droplets. This is because the ice bridges are fed by water vapor evaporating from the droplets. If one can spread the droplets far enough from one another, it’s possible for a droplet to evaporate completely before the ice bridge reaches it, thereby disrupting the spread of frost. (Video credit: J. Boreyko et al.; research paper)

Supercooling Water
Supercooling is the process of lowering a fluid’s temperature below its freezing point without the fluid becoming solid. Though this may sound bizarre, it’s an effect you can recreate easily in your refrigerator, as detailed in the video above. Supercooling shows up in nature as well, particularly with water droplets at high altitudes. If a plane flies through supercooled water droplets, it can create icing problems on the aircraft’s wings. Alternatively, flying through supercooled water vapor can cause a hole-punch cloud to form when the vapor flash-freezes into snow. (Video credit: SciShow)

The Evolution of Icicles
The time-lapse video above shows the growth of icicles of various compositions under laboratory conditions. Many icicles in nature exhibit a rippling effect in their shape, which some theories attribute to an effect of lower surface tension in some liquids. Here researchers show the icicle growth of three liquids: pure distilled water, and water with two concentrations of dissolved salt. They found that lowering the surface tension of the freezing liquid with non-ionic surfactants (i.e. not salt) did not produce ripples, but that dissolved ionic impurities like salt strongly affected the growth of ripples. They posit that this may be due to constitutional supercooling, in which growth of the solid-liquid interface is destabilized by the preferential concentration of impurities near the interface. (Video credit: A. S. Chen and S. Morris)

“Cascades”
Ryan Teague’s “Cascades” music video features the enchanting process of ice growth. A chamber full of supercooled water vapor subject to a strong electric field is stimulated to grow crystals by providing a needle as the initial nucleation site. Because the vapor is supercooled, it will freeze upon contact with the nucleation site; the electric field keeps the water molecules aligned so that the crystal patterns formed are more even. The tree-like pattern seen here is called dendritic crystal growth; branches form at faults in the crystalline pattern. (Video credit: Ryan Teague, Village Green, Words are Pictures; via Gizmodo)