A few years ago, researchers described how an alcohol-water droplet atop an oil bath could pull itself apart through surface tension forces. Dubbed Marangoni bursting, this phenomena has shown up several times since. Here, researchers explore a twist on the behavior by adding surfactants to see how they affect the bursting phenomenon. (Video and image credit: K. Wu and H. Stone; via GFM)
Tag: surfactant

Tweaking Coalescence
When a drop settles gently against a pool of the same liquid, it will coalesce. The process is not always a complete one, though; sometimes a smaller droplet breaks away and remains behind (to eventually do its own settling and coalescence). When this happens, it’s known as partial coalescence.
Here, researchers investigate ways to tune partial coalescence, specifically to produce more than a single droplet. To do so, they add surfactants to the oil layer surrounding their water droplet. The surfactants make the rebounding column of water skinnier, which triggers the Rayleigh-Plateau instability that’s necessary to break the column into more than one droplet. (Image and video credit: T. Dong and P. Angeli)

Lasers and Soap Films
Soap films are a great system for visualizing fluid flows. Researchers use them to look at flags, fish schooling and drafting, and even wind turbines. In this work, researchers explore the soap film’s reaction to lasers. When surfactant concentrations in the soap film are low, laser pulses create shock waves (above) in the film that resemble those seen in aerodynamics. The laser raises the temperature at its point of impact, lowering the local surface tension. That temperature difference triggers a Marangoni flow that draws the heated fluid outward. The low surfactant concentration gives the soap film relatively high elasticity, and that allows the shock waves to form.
In contrast, a soap film with a high concentration of surfactants has relatively little elasticity. In these films (below), the laser creates a mark that stays visible on the flowing soap film. This “engraving” technique could be used to visualize flow in the soap film without using tracer particles. (Image and research credit: Y. Zhao and H. Xu)

When surfactant concentrations are high, a laser pulse “engraves” spots onto a flowing soap film. Shown in terms of interference (left) and Schlieren (right) imaging. 
Surfactants and Waves
In the ocean, waves often curl over and trap air, becoming plunging breakers. How do surfactants like soap or oil affect this process? That’s the question behind this video, where researchers visualize breaking waves with differing amounts of added surfactant. In the case of pure water, the wave forms a smooth jet that curls over and traps air when the wave breaks. As more and more surfactant gets added, the shape of that jet and cavity change. In one case, they become irregular. In another, they disappear entirely, and with the most surfactant added, the wave suddenly looks just like the water-only case.
The key to these behaviors, it turns out, is not how much surfactant there is, but how much the concentration of surfactant varies along the length of the wave. When there are significant changes in the surfactant concentration along the wave, local Marangoni flows try to even out the surface tension, causing the wave to break up in an irregular fashion. (Image and video credit: M. Erinin et al.)

Ink-Based Propulsion
In this video, Steve Mould explores an interesting phenomenon: propulsion via ballpoint pen ink. Placing ink on one side of a leaf or piece of paper turns it into a boat with a dramatic dye-filled wake. It’s not 100% clear what’s happening here, though I agree with Steve that there are likely several effects contributing.
Firstly, there’s the Marangoni effect, the flow that happens from an area of low surface tension to high surface tension. This is what propels a soap boat as well as many water-walking insects. I think this is a big one here, and not just because the ink has surfactants. As any component of the ballpoint ink spreads, its varying concentration is going to trigger this effect.
Secondly, there’s a rocket effect. Rockets operate on a fairly simple principle: throw mass out the back in order to go forward. These dye boats are also doing this to some extent.
And finally there’s some chemistry going on. Some kind of reaction seems to be taking place between one or more of the ink components and the water in order to create the semi-solid layer of dye. Presumably this is why the dye doesn’t simply dissolve as it does in some of Steve’s other experiments.
I figure some of my readers who are better versed in interfacial dynamics, rheology, and surface chemistry than I am will have some more insights. What do you think is going on here? (Video and image credit: S. Mould)

Taking A Turn
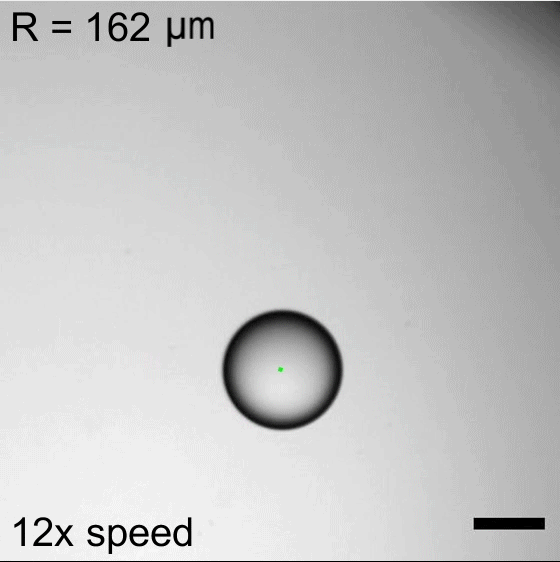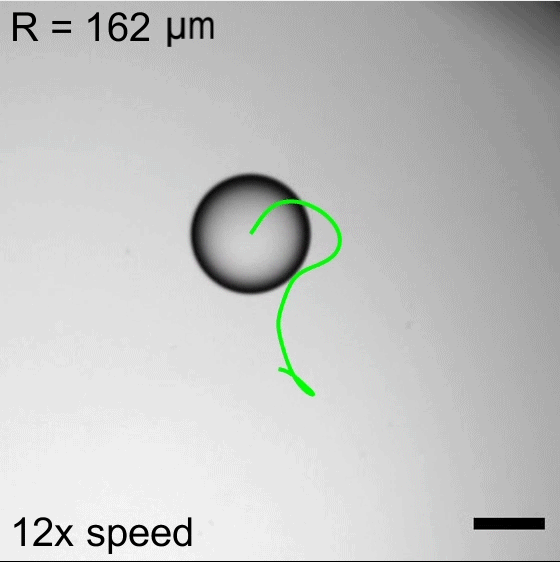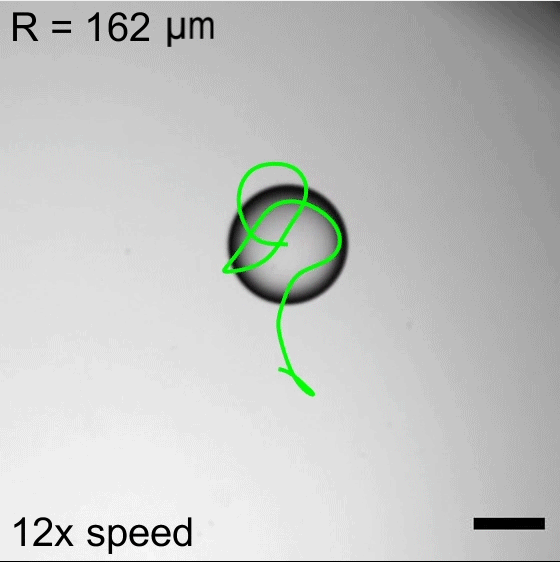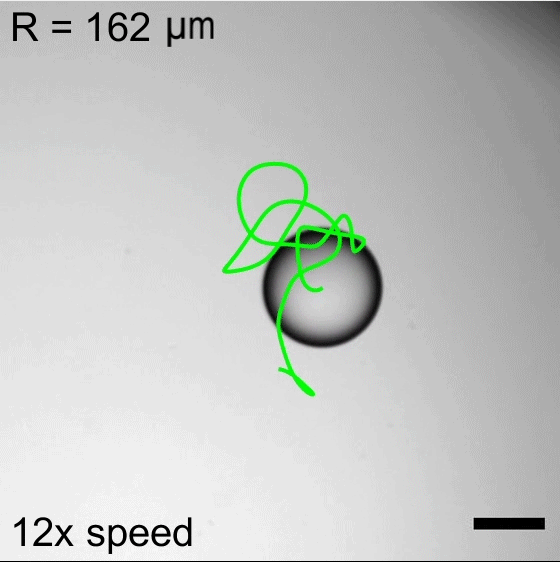
Water droplets immersed in a mixture of oil and surfactants will move about, propelled by the Marangoni effect. Surfactant molecules congregate along the interface between the water and oil, but they do not do so uniformly. This uneven grouping causes variations in the surface tension, which in turn creates flows inside the droplet from areas of low surface tension to ones with higher surface tension. Those internal flows then dictate how the droplet as a whole moves.
Researchers found that droplet trajectories in these systems depend on the droplet’s size. Small droplets move in relatively straight lines, whereas larger droplets take highly curved paths. The difference comes from the way surfactants get distributed around the drop’s interface. Larger drops are more sensitive to shifts in surfactant location, making them more prone to take changeable, curving paths. (Image credits: top – P. Godfrey, others – S. Suda et al.; research credit: S. Suda et al.; via APS Physics)

Stabilizing Foams
Bubbles in a pure liquid don’t last long, but with added surfactants or multiple miscible liquids, bubbles can form long-lasting foams. In soapy foams, surfactants provide the surface tension gradients necessary to keep the thin liquid layers between bubbles from popping. But what stabilizes a surfactant-free foam?
New work finds that foams in mixtures of two miscible fluids only form when the surface tension depends nonlinearly on the concentration of the component liquids. When this is true, thinning the wall between bubbles creates changes in surface tension that stabilize the barrier and keep it from popping.
In mixtures without this nonlinearity, foams just won’t form. The new results are valuable for manufacturing, where companies can avoid unintentional foams simply by careful selection of their fluids. (Image credit: G. Trovato; research credit: H. Tran et al.; via APS Physics; see also Ars Technica, submitted by Kam-Yung Soh)

Oil Drops and Filter Feeders
Natural oils provide critical nutrients to filter feeders like zooplankton and barnacles. These creatures capture oil droplets on bristle-like appendages such as cilia and setae. But this droplet-catching turns into a disadvantage during petroleum spills, when capturing and ingesting oil can be lethal. A recent study looks at the fluid dynamics of oil droplet capture for these tiny creatures.
The authors found that filter feeders capture a range of droplets regardless of size and oil viscosity. But not all droplets stay attached long enough to get consumed, and the larger a droplet is, the lower the flow velocity necessary to detach it from the animal. That suggests a method of limiting uptake of spilled petroleum into the marine food chain: use surfactants to break up the oil into droplets large enough that they’ll detach from filter feeders before getting eaten. (Image credit: D. Pelusi; research credit: F. Letendre et al.; submitted by Christopher C.)

Polygonal Droplets
Spheres are a special shape; they provide the smallest possible surface area necessary to contain a given volume. And since surface tension tries to minimize surface energy by reducing the surface area, drops and soap bubbles are, generally, spherical. There’s subtlety here, though: namely, what if reducing the surface area doesn’t minimize the surface energy?
That’s the issue at the heart of this study. It looks at microscale oil droplets, like the ones above, that are floating in water and stabilized by surfactants. We’d expect droplets like these to be round, and above a critical temperature, they are. But as the temperature drops, the surfactant molecules along the droplet’s interface crystallize. The drop itself is still liquid, but interface is not.
This changes the rules of the game. There’s no way for the surfactant molecules to form a sphere when solidified; they simply can’t fit together that way. So instead defects form along the interface and the drop becomes faceted. As the temperature drops further, the energy relationship between the water, oil, and surfactants continues shifting, causing the droplet to change shape – even to increase its surface area – all to minimize the overall energy. The effect is reversible, too. Raise the temperature back up above the critical point, and the interface “thaws” so that the drop becomes round again. (Image and research credit: S. Guttman et al.; via Forbes; submitted by Kam-Yung Soh)

Exploding a Drop
Leidenfrost drops levitate over a hot substrate on a thin layer of their own vapor, constantly replenished as the drop evaporates. For the most part, previous studies have focused on pure droplets, but a new one looks at what happens when you add surfactants – and the results are, well, explosive.
Surfactants are a type of chemical that like to gather at the surface of a drop, and, unlike water, they’re nonvolatile – they don’t evaporate easily. So as the Leidenfrost drop evaporates and shrinks, the surface of the drop becomes more and more crowded with surfactant molecules. Eventually, they form an elastic shell around the remaining water, making evaporation more difficult.
Inside the droplet, the temperature continues to rise, eventually reaching a point where bubbles of vapor can nucleate inside. When that happens, the bubbles expand almost instantaneously and the internal pressure spike bursts the shell, causing the entire droplet to explode. (Image and research credit: F. Moreau et al.)


![Black and white image of a film pulled outward and breaking into droplets. Text reads, "The [0.05%] surfactant renders the ejected droplets prone to 'popping'." Black and white image of a film pulled outward and breaking into droplets. Text reads, "The [0.05%] surfactant renders the ejected droplets prone to 'popping'."](https://fyfluiddynamics.com/wp-content/uploads/surfburst2-1024x576.png)
![Black and white image of a film pulled outward and spreading in unevenly. Text reads, "When surfactant concentration is further increased [to 1%], drop spreading resumes." Black and white image of a film pulled outward and spreading in unevenly. Text reads, "When surfactant concentration is further increased [to 1%], drop spreading resumes."](https://fyfluiddynamics.com/wp-content/uploads/surfburst3-1024x576.png)