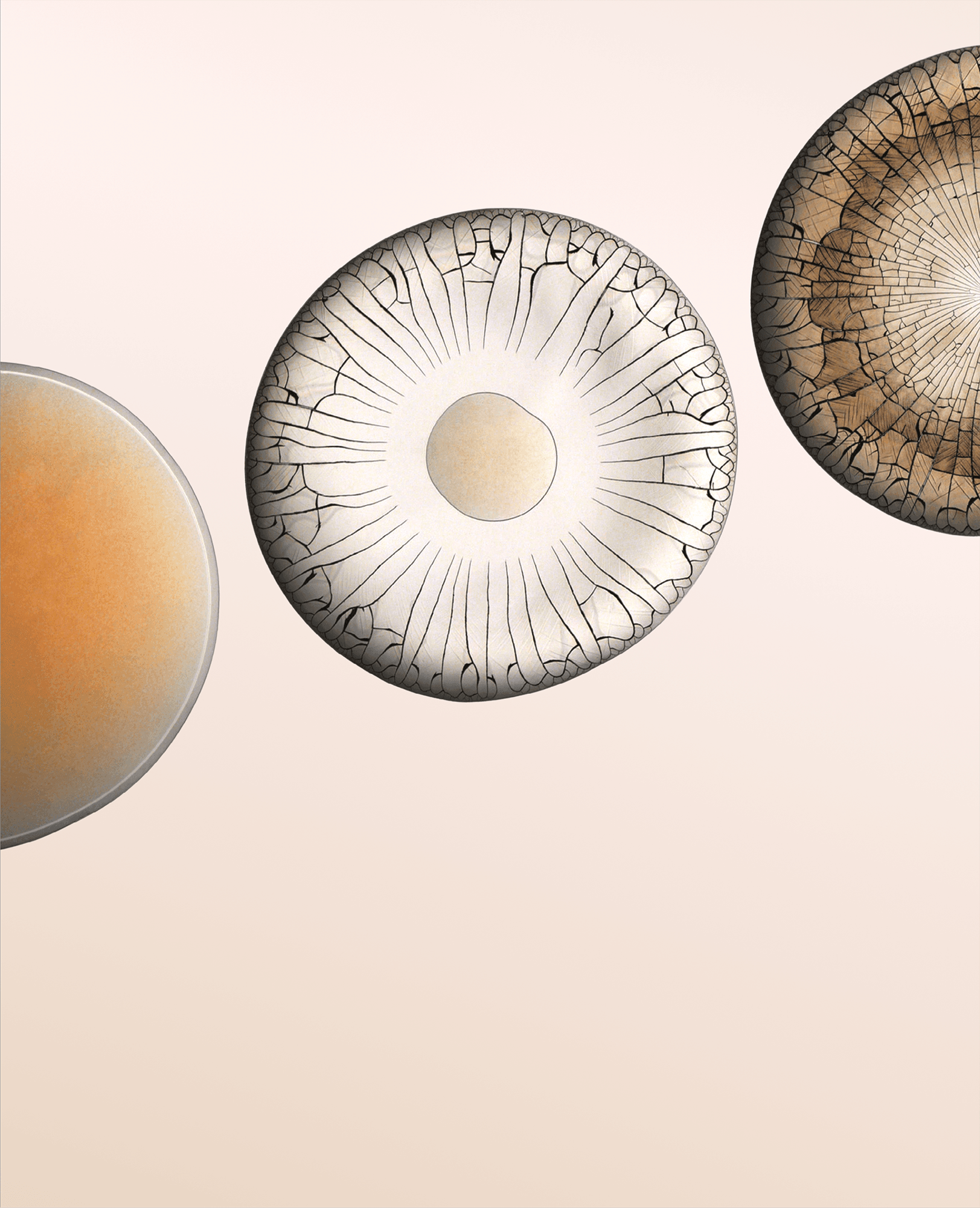Ngangla Ringco sits atop the Tibetan Plateau, breaking up the barren landscape with eye-catching teal and blue. This saline lake sits at an altitude of 4,700 meters, fed by rainfall, Himalayan runoff, and melting glaciers and permafrost. The lake, like many inland bodies of salt water, has no outflow. Instead, water evaporates from the lake, leaving behind any salts that were dissolved in it. Over time, those left-behind salts build up and make the lake ever saltier. (Image credit: NASA; via NASA Earth Observatory)
Tag: evaporation

Dissolution and Crystallization
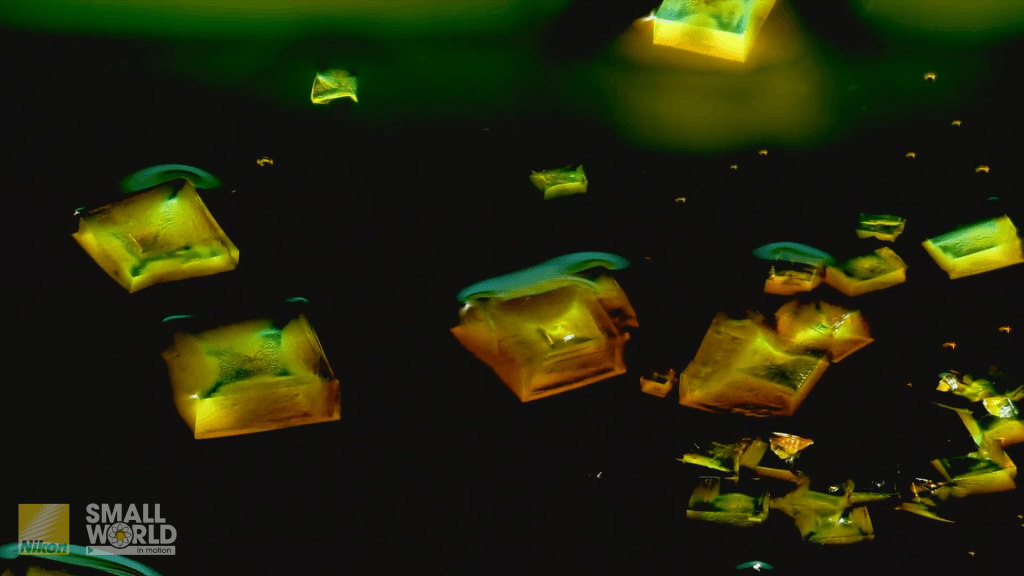
A colorful assortment of salts dissolve and recrystallize in this microscopic timelapse video by retired engineer Jay McClellan. Every step is a gorgeous rainbow of color as the cobalt, copper, and sodium chlorides dissolve, mix, and change. Though we don’t see what’s going on in the water, fluid dynamics are a critical component of both dissolution and crystallization. In the former, concentration gradients change the water’s density, driving buoyant flows. For the latter, crystallization comes out of evaporation, where surface tension often determines where solid particles get left behind. (Video and image credit: J. McClellan; via Colossal)

Evaporating Off Butterfly Scales
This award-winning macro video shows scattered water droplets evaporating off a butterfly‘s wing. At first glance, it’s hard to see any motion outside of the camera’s sweep, but if you focus on one drop at a time, you’ll see them shrinking. For most of their lifetime, these tiny drops are nearly spherical; that’s due to the hydrophobic, water-shedding nature of the wing. But as the drops get smaller and less spherical, you may notice how the drop distorts the scales it adheres to. Wherever the drop touches, the wing scales are pulled up, and, when the drop is gone, the scales settle back down. This is a subtle but neat demonstration of the water’s adhesive power. (Video and image credit: J. McClellan; via Nikon Small World in Motion)

Water droplets evaporate from the wing of a peacock butterfly. 
“C R Y S T A L S”
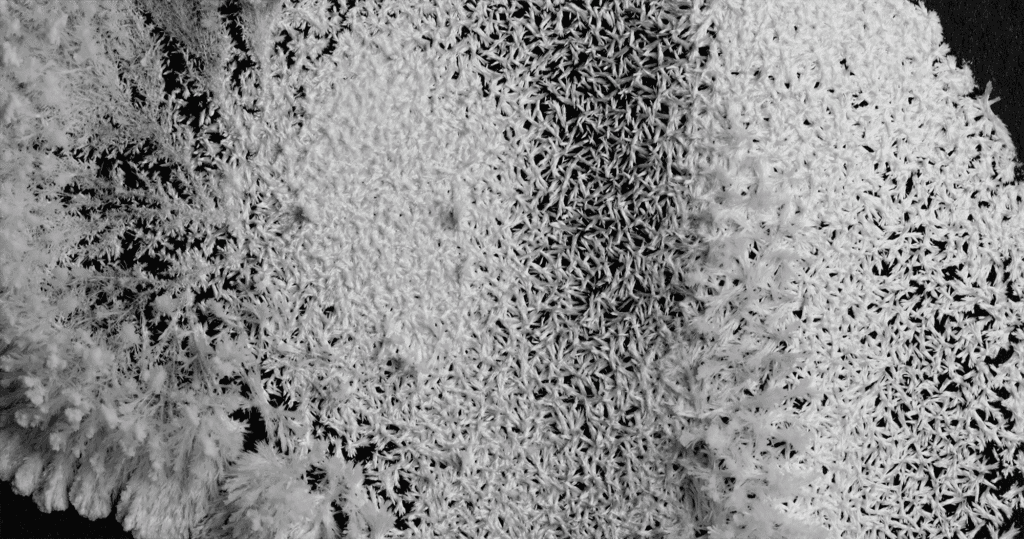
In “C R Y S T A L S,” filmmaker Thomas Blanchard captures the slow, inexorable growth of potassium phosphate crystals. He took over 150,000 images — one per minute — to document the way crystals formed as the originally transparent liquid evaporated. Some crystals branch into fractals. Others bulge outward like a condensing cloud or a sprouting mushroom. (Video and image credit: T. Blanchard)

“Monsoon 7”
Storm-chasing photographer Mike Olbinski (previously) returns with another stunning timelapse of summer thunderstorms in the western U.S. I never tire of watching the turbulent convection, microbursts, billowing haboobs, and undulating clouds Olbinski captures. His work is always a reminder of the incredible power and energy contained in our atmosphere and unleashed in cycles of warming and cooling, evaporation and condensation. (Video and image credit: M. Olbinski)

“My Own Galaxy”
Fungal spores sketch out minute air currents in this shortlisted photograph by Avilash Ghosh. The moth atop a mushroom appears to admire the celestial view. In the largely still air near the forest floor, mushrooms use evaporation and buoyancy to generate air flows capable of lifting their spores high enough to catch a stray breeze. (Image credit: A. Ghosh/CUPOTY; via Colossal)

The Mystery of the Binary Droplet
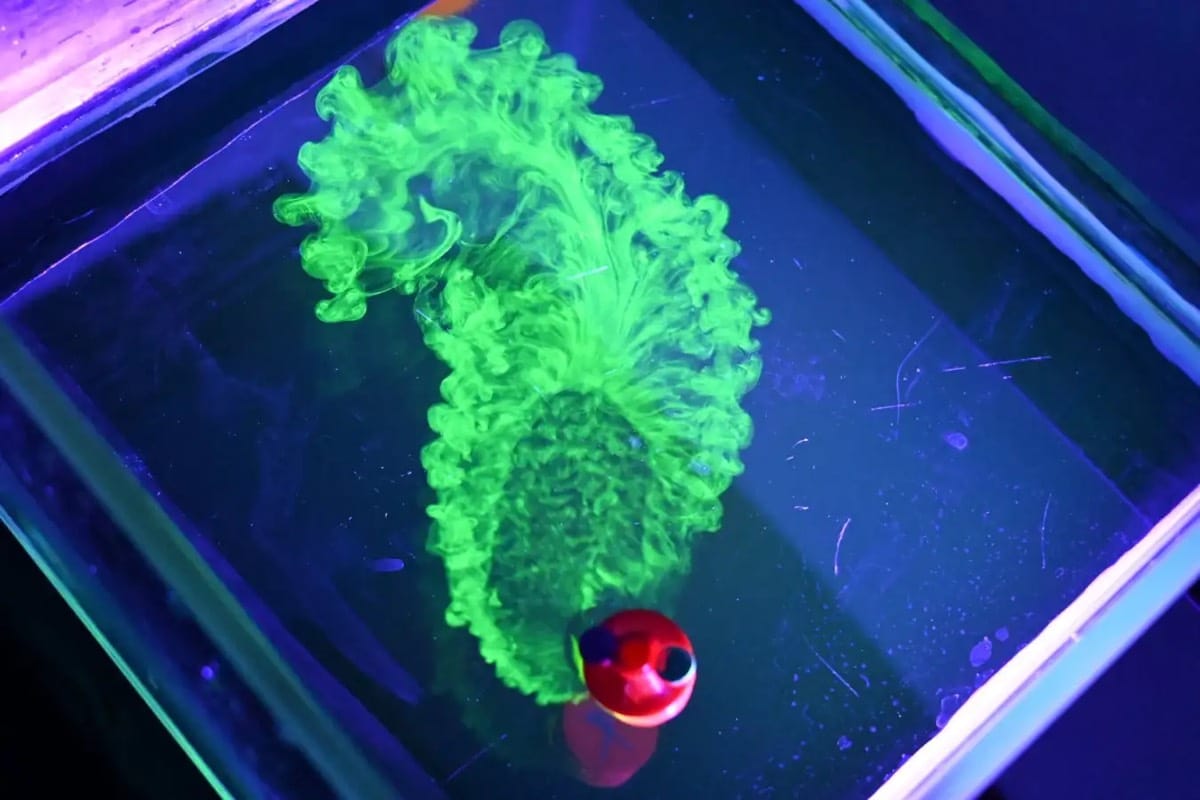
What goes on inside an evaporating droplet made up of more than one fluid? This is a perennially fascinating question with lots of permutations. In this one, researchers observed water-poor spots forming around the edges of an evaporating drop, almost as if the two chemicals within the drop are physically separating from one another (scientifically speaking, “undergoing phase separation“). To find out if this was really the case, they put particles into the drop and observed their behavior as the drop evaporated. What they found is that this is a flow behavior, not a phase one. The high concentration of hexanediol near the edge of the drop changes the value of surface tension between the center and edge of the drop. And that change is non-monotonic, meaning that there’s a minimum in the surface tension partway along the drop’s radius. That surface tension minimum is what creates the separated regions of flow. (Video and image credit: P. Dekker et al.; research pre-print: C. Diddens et al.)

How Cooling Towers Work
Power plants (and other industrial settings) often need to cool water to control plant temperatures. This usually requires cooling towers like the iconic curved towers seen at nuclear power plants. Towers like these use little to no moving parts — instead relying cleverly on heat transfer, buoyancy, and thermodynamics — to move and cool massive amounts of water. Grady breaks them down in terms of operation, structural engineering, and fluid/thermal dynamics in this Practical Engineering video. Grady’s videos are always great, but I especially love how this one tackles a highly visible piece of infrastructure from multiple engineering perspectives. (Video and image credit: Practical Engineering)

Active Cheerios Self-Propel
The interface where air and water meet is a special world of surface-tension-mediated interactions. Cereal lovers are well-aware of the Cheerios effect, where lightweight O’s tend to attract one another, courtesy of their matching menisci. And those who have played with soap boats know that a gradient in surface tension causes flow. Today’s pre-print study combines these two effects to create self-propelling particle assemblies.
The team 3D-printed particles that are a couple centimeters across and resemble a cone stuck atop a hockey puck. The lower disk area is hollow, trapping air to make the particle buoyant. The cone serves as a fuel tank, which the researchers filled with ethanol (and, in some cases, some fluorescent dye to visualize the flow). Like soap, ethanol’s lower surface tension disrupts the water’s interface and triggers a flow that pulls the particle toward areas with higher surface tension. But, unlike soap, ethanol evaporates, effectively restoring the interface’s higher surface tension over time.
With multiple self-propelling particles on the interface, the researchers observed a rich series of interactions. Without their fuel, the Cheerios effect attracted particles to each other. But with ethanol slowly leaking out their sides, the particles repelled each other. As the ethanol ran out and evaporated, the particles would again attract. By tweaking the number and position of fuel outlets on a particle, the researchers found they could tune the particles’ attractions and motility. In addition to helping robots move and organize, their findings also make for a fun educational project. There’s a lot of room for students to play with different 3D-printed designs and fuel concentrations to make their own self-propelled particles. (Research and image credit: J. Wilt et al.; via Ars Technica)