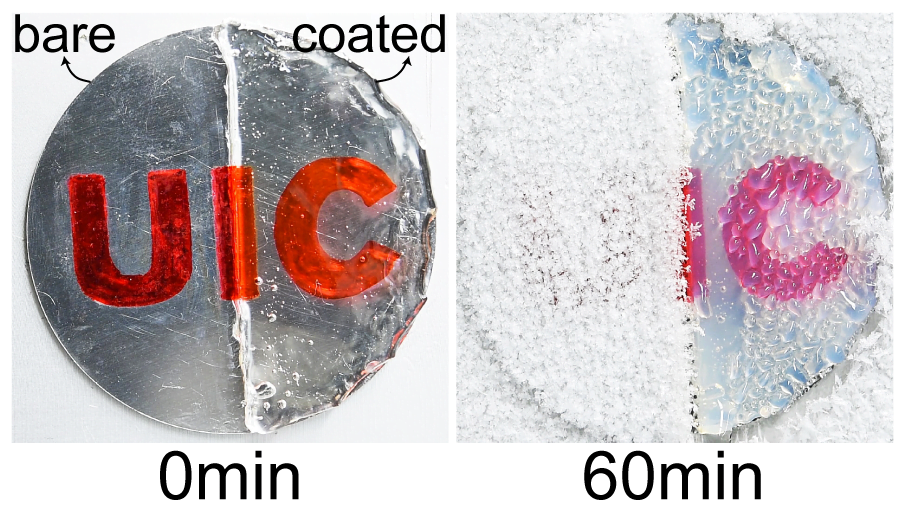
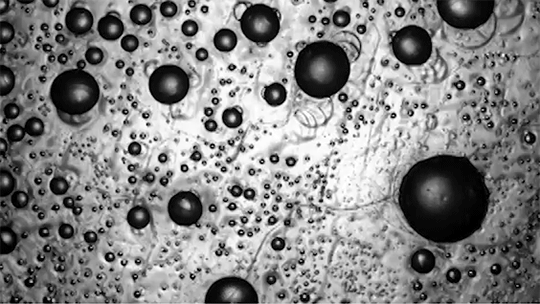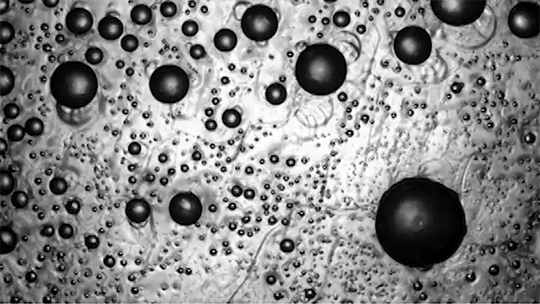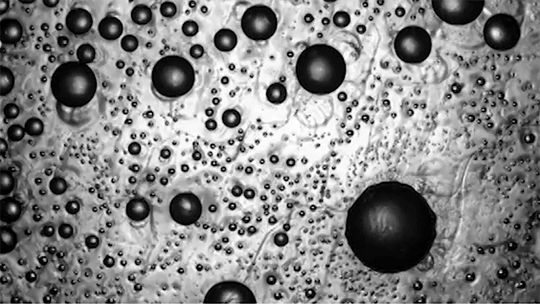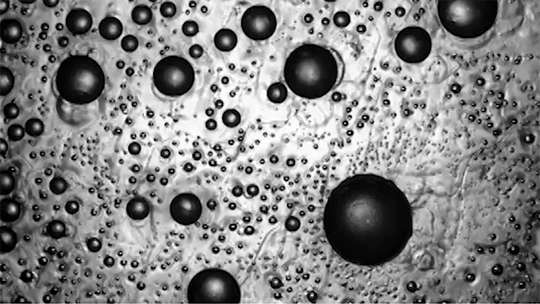
Despite spending their lives in and around frigid water, snow, and ice, polar bears are rarely troubled by ice building up on their fur. This natural anti-icing property is one Inuits have long taken advantage of by using polar bear fur in hunting stools and sandals. In a new study, researchers looked at just how “icephobic” polar bear fur is and what properties make it so.
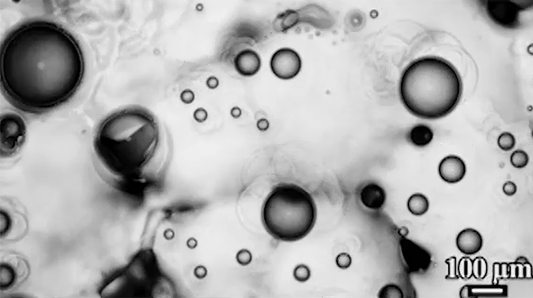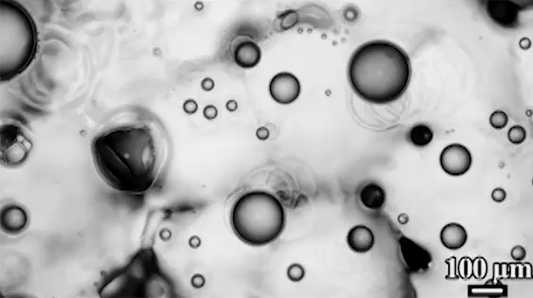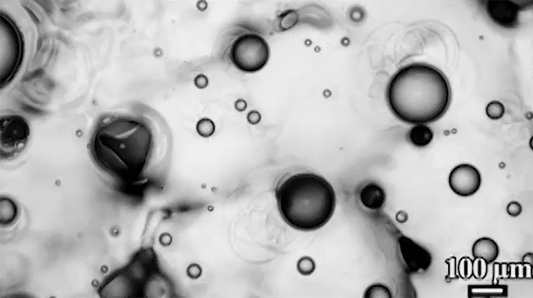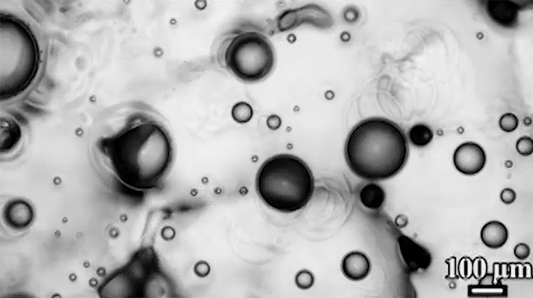
The key to a polar bear’s anti-icing is sebum — a mixture of cholesterol, diacylglycerols, and fatty acids secreted from glands near each hair’s root. When sebum is present on the hair, the researchers found it takes very little force to remove ice; in contrast, fur that had been washed with a surfactant that stripped away the sebum clung to ice.
The researchers are interested in uncovering which specific chemical components of sebum impart its icephobicity. That information could enable a new generation of anti-icing treatments for aircraft and other human-made technologies; right now, many anti-icing treatments use PFAS, also known as “forever chemicals,” that have major disadvantages to human and environmental health. (Image credit: H. Mager; research credit: J. Carolan et al.; via Physics World)