Even freshwater contains trace salts and minerals that cause scaly buildups as they evaporate. Getting rid of the scale usually requires toxic chemicals and/or lots of scrubbing, neither of which are desirable at the industrial level. At the same time, we’re extremely limited in the amount of freshwater that we have available; only about 1% of Earth’s water is liquid and fresh. If we could use salt water in more industrial processes, that would preserve freshwater for drinking and agriculture. But how do we tackle the scaly buildup?

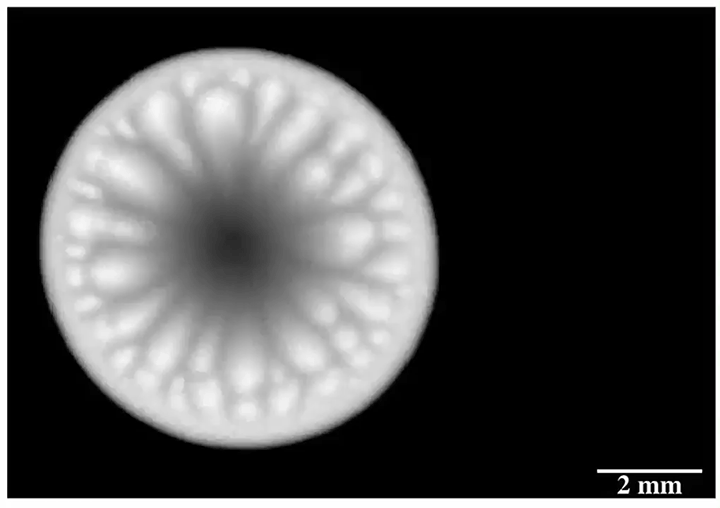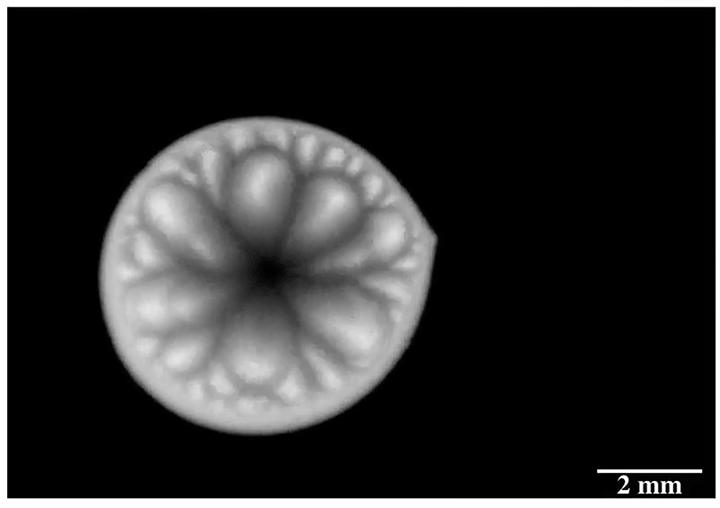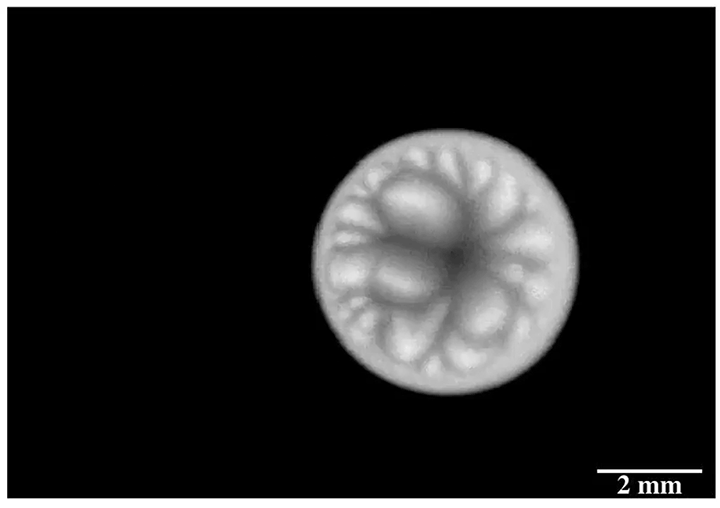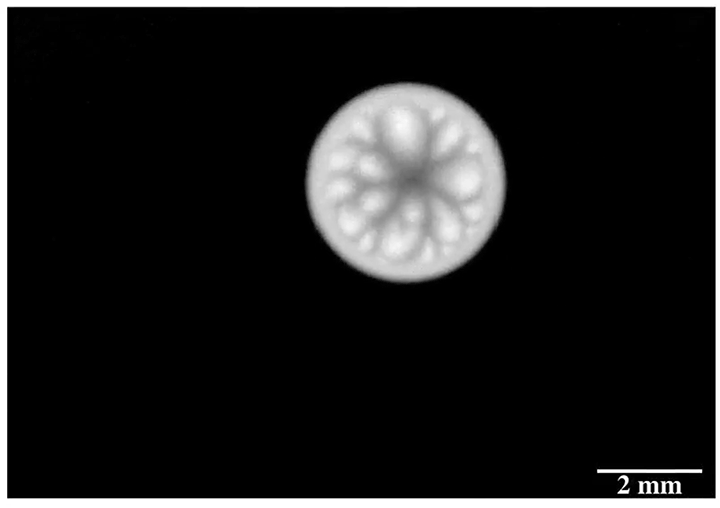
Enter “salt critters.” Researchers found that when salt water evaporated from microtextured surfaces designed to shed water, salt would eventually build up in the gaps, breaking the hydrophobic effect and allowing scale to build up. In contrast, a nanotextured surface left nowhere for the salt to adhere. On these surfaces, evaporating salt water built jellyfish-like salt critters that rose from the surface and, eventually, broke off and rolled away, leaving the surface pristine. (Image credit: S. McBride; research credit: S. McBride et al.; via Physics Today)