Here’s another look at SurferBot, a low-cost, vibration-based robot capable of traversing both water and land. SurferBot’s vibration creates asymmetric ripples on the water surface. Because the waves are bigger at the rear of the robot, it gets propelled forward. But there doesn’t have to be water for SurferBot to get around! It’s actually amphibious, moving on both land and water. It can even transition from land to water on its own. (Image and video credit: E. Rhee et al.; research credit: E. Rhee et al.)
Search results for: “transition”

Why Moths Are Slow Fliers
Hawkmoths and other insects are slow fliers compared to birds, even ones that can hover. To understand why these insects top out at 5 m/s, researchers simulated their flight from hovering to forward flight at 4 m/s. They analyzed real hawkmoths flying in wind tunnels to build their simulated insects, then studied their digital moths with computational fluid dynamics.
During hovering flight, they found that hawkmoths generate equal amounts of lift with their upstroke and downstroke. As the moth transitions into forward flight, though, its wing orientation shifts to reduce drag, and the upstroke stops being so helpful. Instead, the upstroke generates a downward lift that the downstroke has to counter in addition to the insect’s weight. At higher forward speeds, this trend gets even worse.
The final verdict? Hawkmoths don’t have the flexibility to twist their wings on the upstroke the way birds do to avoid that large downward lift. Since they can’t mitigate that negative lift, the insects have a slower top speed overall. (Image and research credit: S. Lionetti et al.; via APS Physics; submitted by Kam-Yung Soh)
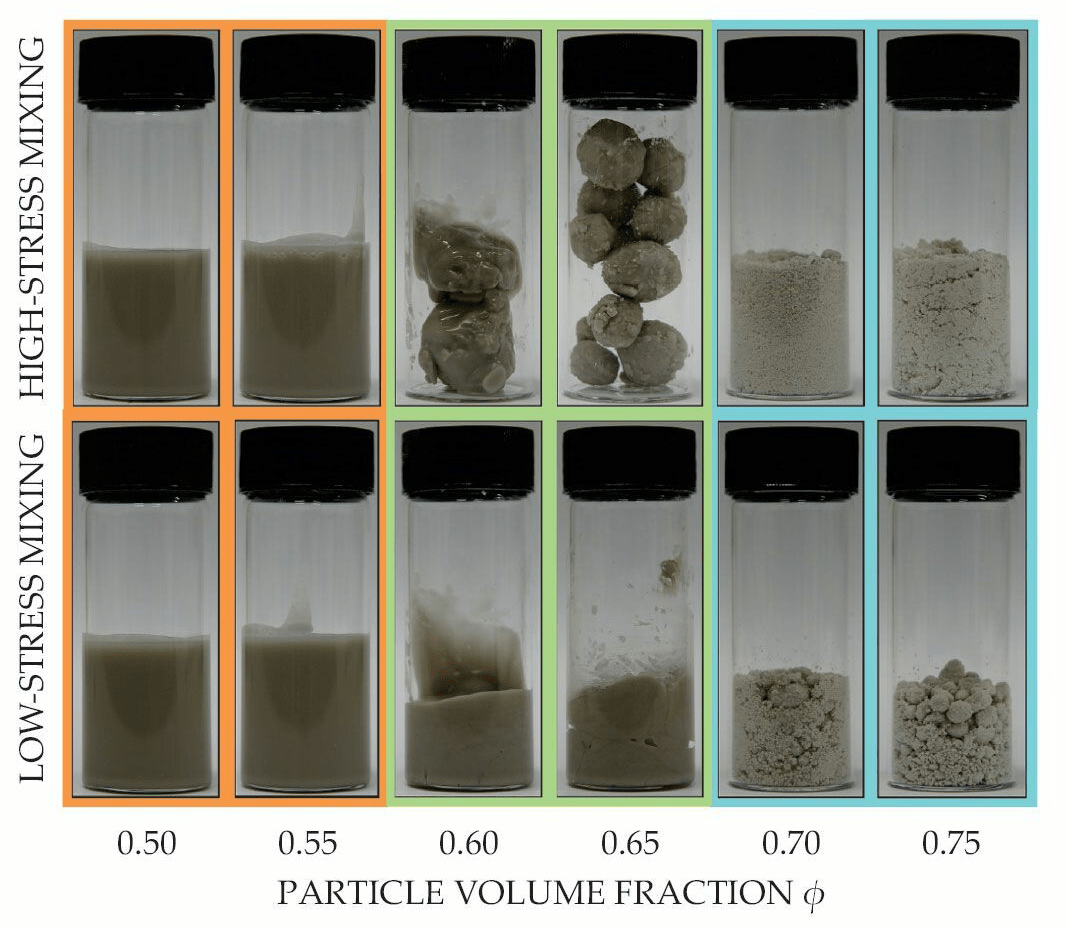
Mixing the Perfect Batter
In baking, there’s a point when wet and dry ingredients get combined to form the batter (or dough) that eventually becomes a tasty treat. Experienced bakers know that the ratio of wet-to-dry must be just right for the final product. Too dry and the mixture won’t come together; too wet and the final product is a soggy mess.
Mixing liquids and powders is ubiquitous outside the kitchen, too. Ceramics, concrete, laundry detergent, chocolate — all involve this critical step. To understand how these mixtures transition from fluid to clustered granules to granulations (think wet sand), researchers carefully studied a mixture of glass spheres and glycerol. When there were relatively few particles in the mixture (in technical terms, a smaller “particle volume fraction”), the mixture was fully fluid (top image, orange background). When the ratio of particles-to-liquid was high, the mixture was granular (blue background). And in-between these ratios, whether the mixture formed clumps, or granules, depended on how it was mixed (green background). Vigorous mixing (top row) formed large granules, which consisted of a wet, jammed interior and an outer layer of dry particles (lower image).
Their observations allowed the researchers to predict what ratio of liquid and powder is needed, and how much mixing is necessary, to create a desired outcome. (Image and research credit: D. Hodgson et al.; via Physics Today)

A cross-section of a granule, showing the wet, jammed interior (left) surrounded by a region of dry particles (center, enclosed between red dashes). 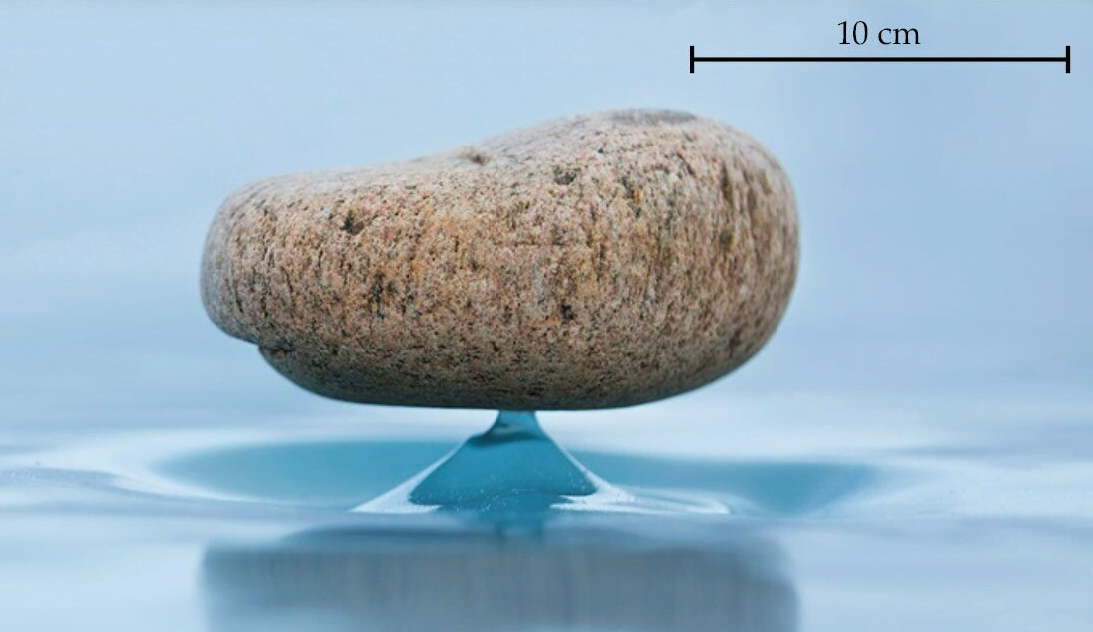
Zen Stones
On Lake Baikal, where Siberian winters are long and cold but have little precipitation, you can find a strange phenomenon: stones that balance on a thin spire of ice. Known as Zen stones — thanks to their visual similarity to stacks of balanced stones in Japanese Zen gardens — these natural oddities rely on time and sublimation, a transition from ice to vapor without melting.
The process is simple. Toss a stone on the ice and wait. As the sun shines, the ice will sublimate, transforming from ice directly to vapor at an estimated rate of ~2 mm per day, for Lake Baikal’s typical weather. But the stone’s presence acts like an umbrella, protecting some of the ice beneath it from the sunlight that is critical for sublimation. As a result of this umbrella effect, a thin column of ice remains beneath the stone.
In the lab, researchers were able to recreate the process in less time by tweaking the temperature, humidity, and irradiance to enhance sublimation. Instead of stones, they used metal disks, but their Zen stones made their ice columns just the same. (Image and research credit: N. Taberlet and N. Plihon; via Physics Today)

A lab Zen stone, formed from a disk of aluminum atop a column of ice. 
Peering Into the Gap
This video offers a glimpse into turbulence developing in a classic flow set-up, a Taylor-Couette cylinder. The apparatus consists of two upright, concentric cylinders; the outer cylinder is fixed, and the inner one rotates. This video shows the gap between the cylinders, and it’s rotated so that the inner cylinder is at the bottom of the frame. Gravity points from left to right in the video. The fluid in the 8-cm gap between the cylinders is water, seeded with rheoscopic particles to visualize the flow.
The video begins as the inner cylinder has just begun to rotate, dragging nearby fluid with it. A thin, laminar boundary layer forms at the bottom of the frame, growing as time goes on. A few seconds in, the boundary layer transitions to turbulence; look closely and you’ll see hairpin-shaped vortices appear. Just after that, the boundary layer becomes entirely turbulent and continues to slowly move upward to take over the full gap. The video is available in a full 4K resolution if you really want to get lost in the flow. (Video credit: D. van Gils)

A Levitated Boil
When acoustically levitated, objects tend to clump together and move like a single, large solid. But researchers found more fluid-like states for their levitated particles when the particles were smaller. At low acoustic power, the particles behave like a liquid and shift primarily within a plane. But as the acoustic power increases, the granular liquid begins to “boil” and transition into a gaseous state, with particles moving in all directions. It’s amazing how often these metaphors (e.g., treating a group of particles as a “liquid”) hold true when observing different physical systems! (Image and video credit: B. Wu et al.)

Quantum Instability
In our everyday lives, two fluids moving past one another often form a wave-like pattern thanks to the Kelvin-Helmholtz instability. We see it in the curl of waves on the ocean, in clouds in the sky, and even in spirals of lava on Mars. Here researchers explore an analogous instability in the quantum world.
By spinning a gas of ultracold atoms, the team observed a spontaneous transition from a needle-like configuration to a crystal made up of spirals. It’s a quantum Kelvin-Helmholtz instability! The authors found that wave’s phase is random; it arises purely from quantum interactions between the atoms. (Image, research, and submission credit: B. Mukherjee et al.; see also MIT News)

The spinning cloud of ultracold atoms breaks up into a series of spirals. 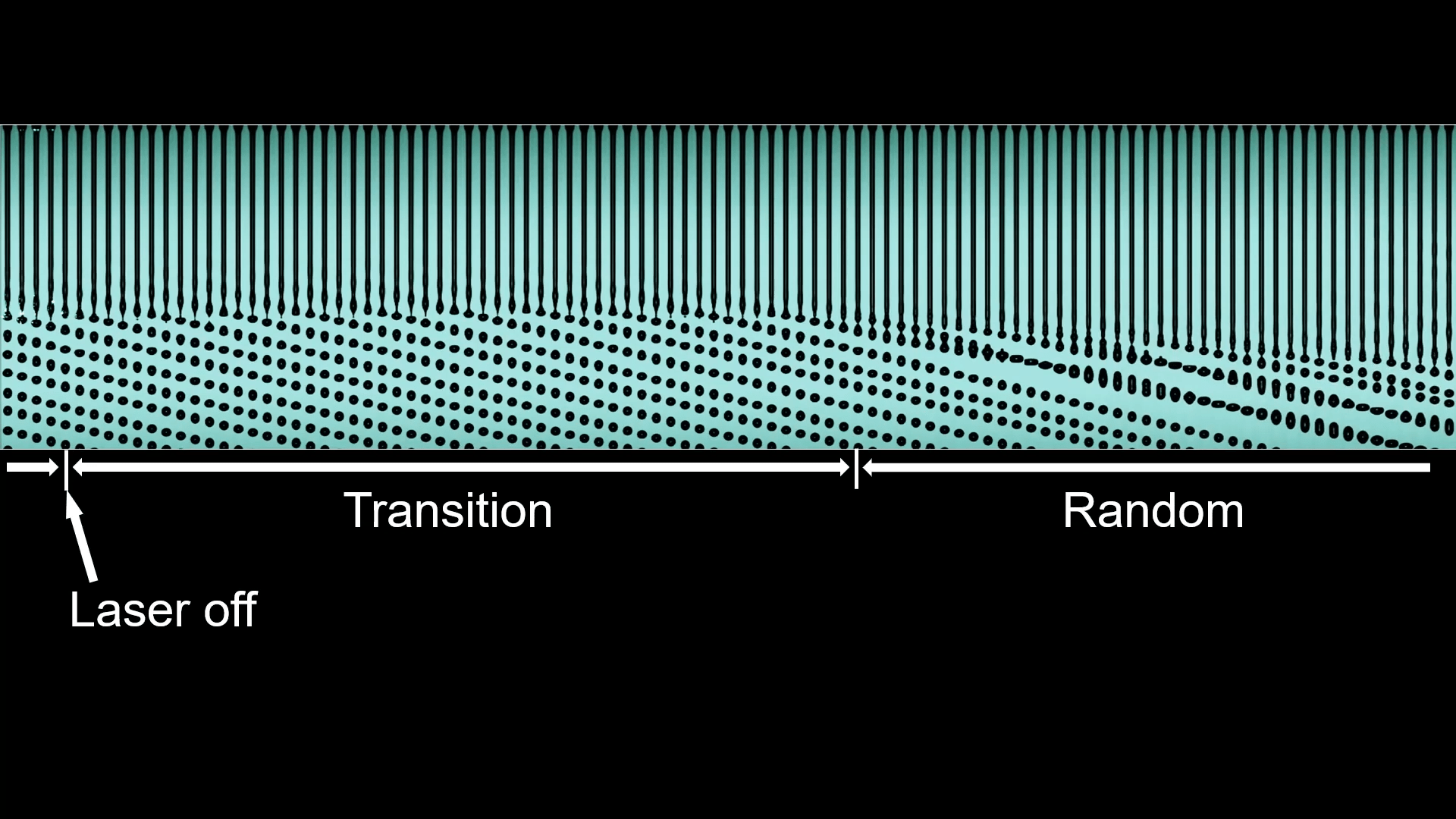
Laser-Induced Jet Break-Up
A falling stream of water will naturally break up into droplets via the Plateau-Rayleigh instability. Those droplets are random, unless something like vibration of the nozzle sets their size. In this study, though, researchers found that shining a laser beam on the stream can trigger an orderly break-up with droplets that are consistent in size and spacing.
The optofluidic phenomenon depends on a few different effects. The changing curvature of the liquid stream reflects the laser light, some of which undergoes total internal reflection and travels up the jet as if it were a fiber optic cable. Look closely in the right side of the second image, and you’ll see a periodic flicker of green light at the mouth of the nozzle. Those flashes of green reveal that the liquid jet is guiding the light upstream in bursts, each of which exerts an optical pressure that triggers the Plateau-Rayleigh instability.
When the laser first turns on, there’s a transition period before the orderly break-up begins, and, likewise, turning the laser off triggers a transition from orderly to random (top image). (Image and research credit: H. Liu et al.; via APS Physics; submitted by Kam-Yung Soh)

A Colorful Fire Tornado
This one definitely belongs in the do-not-try-this-yourself category, but this Slow Mo Guys video of a colorful fire tornado is pretty spectacular. Using an array of different fuels and a ring of box fans, Gav sets up a vortex of flame that transitions smoothly from red all the way to blue. As he points out in the video, the translucency of the vortex is so good that you can see how the two sides of the vortex rotate! (Video credit: The Slow Mo Guys)

Flying Out of the Water
Flying fish and diving birds often navigate the interface between water and air in their flight, but few studies have actually looked at the effects of this transition on lift. In this work, researchers measured forces on a small, fixed wing as it egresses from water into air at a constant velocity.

The tests showed that exit velocity had a large effect on lift generation. At low speeds, an exiting wing experienced a strong, positive lift spike as soon as the leading edge broke the surface. But that lift changed to strongly negative as the wing continued out of the water. At higher speeds, the wings had no lift reversal but also reached lower peak lift coefficients. The team studied the effects of angle of attack and starting depth as well, concluding that any vehicles intended to navigate the water-air transition will need robust control systems prepared to deal with fast-changing forces. (Image credit: fish – J. Cobb, wing – W. Weisler et al.; research credit: W. Weisler et al.)















