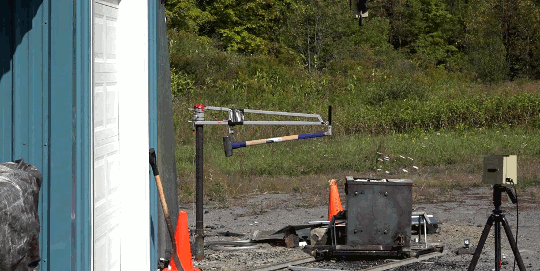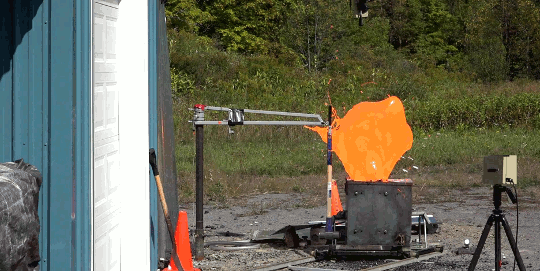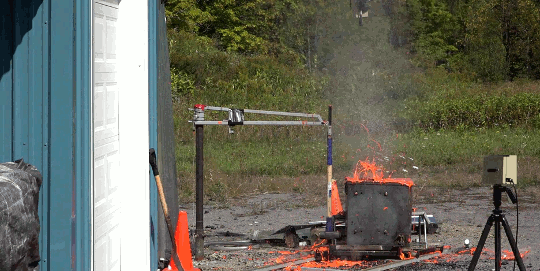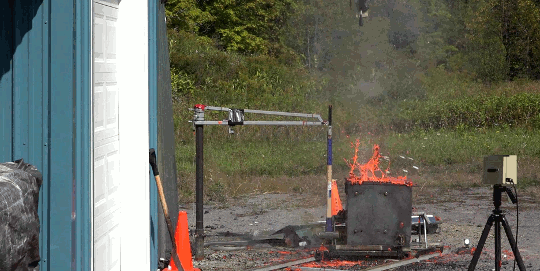
As part of the demolition of a decommissioned coal-fired power plant in Nottinghamshire, workers simultaneously demolished eight cooling towers. The video is here. As the towers collapse, smoke and dust gets blown both out of the base and up each tower. The flow details are fascinating. The plumes have rings in them, perhaps related to how the blast’s waves reflect in the tower or how the structure itself fails. Vortex rings curl up as the rising plumes mix with the surrounding air. If you’re anything like me, you’ll have to replay it several times! (Image credit: BBC; submitted by jshoer)
Tag: explosion

Can Explosions Deflect Bullets?
In one of their most Mythbusters-like videos ever, the Slow Mo Guys ask: can an explosion deflect a bullet? To find out, they built out a system to trigger a C4 explosive using a 9mm bullet, all while watching with a series of high-speed cameras. As you’d expect, there are lots of blast waves and neat flame propagation to watch. As for the fundamental question, well, you’ll have to watch to find out! (Video and image credit: The Slow Mo Guys)

Switchable Explosives
Explosives are used in many fields, including mining and demolition, but storing these devices is difficult and dangerous. Hundreds of accidents — many resulting in fatalities — have happened over the decades, simply because there is no true “off-switch” for explosive devices. But a group out of Los Alamos believe they’ve changed that.

Without water in the device, the outer surfaces burn, but no explosion takes place. Using 3D-printing, the researchers built an explosive lattice filled with empty voids. With air in these gaps, any attempt to light the explosive fizzle. The outer layers of the explosive burn, but there’s no detonation. It is, relatively speaking, safe for storage.

When the voids are filled with water, the explosive detonates when lit. But once the device is filled with water (or another liquid), the story is different. In this situation, the blast wave propagates and the explosive detonates, releasing 98% more energy than in its “storage” mode. Changing the liquid inside the device can enhance the explosive energy, too, which could allow users to tune the discharge. (Image credit: S. Moses; video and research credit: C. Brown et al.; via APS Physics)

Underwater Explosions and Submarines
In the early days of submarines, it did not take physicists and engineers long to discover how destructive underwater explosions can be. In this Slow Mo Guys video, Gav gives us a glimpse of that destruction using a model submarine in a fish tank and several small explosives. You’ll have to be quick to notice the initial shock waves that ripple through the tank, but the footage captures spectacular detail on some of the slower-moving phenomena. You can see the uneven ripples of the explosion bubble’s surface as it expands. There are some great shots from the front and side showing the bubbly vortex ring that forms when the explosion hits the side of the tank wall (something that wouldn’t happen out in the ocean, of course). You can even catch a glimpse of some unexploded powder streaking out of the explosion. (Image and video credit: The Slow Mo Guys)

Explosive Flame Fronts
Though they look like jellyfish or space creatures, these images from photographer Linden Gledhill are actually explosions. What you’re seeing is the detonation of hydrogen gas with oxygen. The teal sphere with its wavy surface marks the flame front, and the crisp, stringy edges seen here and there in the foreground are the remains of a soap bubble that held the hydrogen before it sparked. You can see a similar set-up (using methane rather than hydrogen) in action here, and you can see other artistic takes on combustion in previous posts like this one. (Image credit: L. Gledhill, Flickr)

Exploding a Drop
Leidenfrost drops levitate over a hot substrate on a thin layer of their own vapor, constantly replenished as the drop evaporates. For the most part, previous studies have focused on pure droplets, but a new one looks at what happens when you add surfactants – and the results are, well, explosive.
Surfactants are a type of chemical that like to gather at the surface of a drop, and, unlike water, they’re nonvolatile – they don’t evaporate easily. So as the Leidenfrost drop evaporates and shrinks, the surface of the drop becomes more and more crowded with surfactant molecules. Eventually, they form an elastic shell around the remaining water, making evaporation more difficult.
Inside the droplet, the temperature continues to rise, eventually reaching a point where bubbles of vapor can nucleate inside. When that happens, the bubbles expand almost instantaneously and the internal pressure spike bursts the shell, causing the entire droplet to explode. (Image and research credit: F. Moreau et al.)

Lava Bomb
What you see above is a homemade lava bomb. To systematically study what happens when groundwater meets lava, scientists melted basalt and created their own meter-scale explosion-on-demand. Inside the container, they can inject water and observe the resulting dynamics.
Beneath the lava, the water forms what scientists call a domain. Thanks to the Leidenfrost effect, it can be protected from direct contact with the lava by a thin vapor layer that boils off it. If the water domain is large enough, buoyancy will pull it upward through the lava. Whether the water maintains a spherical shape or begins to distort and break up into smaller domains depends on the speed of its rise.
At some point, though, either naturally or through an external trigger (like the sledgehammer you see above), the water and lava can contact, resulting in explosive vaporization of the water and an explosion. What’s visible at the surface depends on the depth at which the explosion takes place. Scientists are eager to characterize these variations, which will help them better predict the explosive danger of eruptions like Kilauea and Eyjafjallajökull. (Image and research credit: I. Sonder et al.; video credit: NYTimes; submitted by Kam-Yung Soh)

Kilauea’s Lava Lake
Hawaii’s Kilauea Volcano continues to erupt, sending magma flowing through multiple fissures. The U.S. Geological Survey has sounded a warning, however, that the volcano could erupt more explosively. Hot spot volcanoes like Hawaii’s generally have more basaltic lava, which has a lower viscosity than more silica-rich magmas like those seen on continental plates. That makes Hawaii’s volcanoes less prone to explosive detonations like the 1980 Mt. St. Helens eruption. With less viscous lava, there’s less likelihood of plugging a magma chamber and causing a deadly buildup of pressure from toxic gases.
But that doesn’t mean that there’s no risk. In particular, officials are concerned by the rapid draining of a lava lake near Kilauea’s summit. As illustrated below, if the lava level drops below the water table, that increases the likelihood of steam forming in the underground chambers through which lava flows. The rapid drainage has destabilized the walls around the lava lake, causing frequent rockfalls into the chamber. If those were to plug part of the chamber and cause a steam buildup, then there could be an explosive eruption that releases the pressure. To be clear: even if this were to happen, it would be nothing like the explosiveness of Mt. St. Helens. But it would include violent expulsions of rock and widespread ash-fall. (Image credits: USGS, source; via Gizmodo)


Icy Spikes
Water is one of those strange materials that expands when it freezes, which raises an interesting question: what happens to a water drop that freezes from the outside in? A freezing water droplet quickly forms an ice shell (top image) that expands inward, squeezing the water inside. As the pressure rises, the droplet develops a spicule – a lance-like projection that helps relieve some of the pressure.
Eventually the spicule stops growing and pressure rises inside the freezing drop. Cracks split the shell, and, as they pull open, the cracks cause a sudden drop in pressure for the water inside (middle image). If the droplet is large enough, the pressure drop is enough for cavitation bubbles to form. You can see them in the middle image just as the cracks appear.
After an extended cycle of cracking and healing, the elastic energy released from a crack can finally overcome surface energy’s ability to hold the drop together and it will explode spectacularly (bottom image). This only happens for drops larger than a millimeter, though. Smaller drops – like those found in clouds – won’t explode thanks to the added effects of surface tension. (Image credit: S. Wildeman et al., source)
ETA: A previous version of this post erroneously said this was freezing from the “inside out” instead of “outside in”.

Battery Rockets
When I post Slow Mo Guys videos, it often comes with a warning not to try this at home. For their latest video, that deserves an extra-special mention: seriously, don’t try this. In this video, Dan and Gav explode lithium-ion batteries. In the process, they discover a safety feature – namely vents on one face of the battery. Because runaway thermal reactions (a.k.a. explosions) are a possibility with this type of battery system, consumer-grade batteries are designed to try and prevent extreme damage. One of these outwardly visible safety features are these four vents that release gas when when the battery is too hot. By venting the gas, manufacturers keep the battery from exploding and sending hot chemicals and shrapnel in all directions. Instead the venting gas turns the entire battery into a miniature rocket. (Video and image credit: The Slow Mo Guys)