Adding just a little polymer to a pipe flow speeds it up by reducing drag near the wall. But the effects on turbulence away from the wall have been harder to suss out. A new experiment shows that added polymers suppress eddy formation in the flow and reduce how much energy is lost to friction and, ultimately, heat. In particular, the researchers found that polymer stress helped stabilize shear layers in the flow and prevent them from destabilizing into more turbulent flow. (Image credit: S. Wilkinson; research credit: Y. Zhang et al.; via APS)
Tag: polymer effects

Growing Hydrogels in an Active Fluid
Active nematic fluids borrow their ingredients from biology. Using long, rigid microtubules and kinesin motor proteins capable of cross-linking between and “walking” along tubules, researchers create these complex flow patterns. Here, a team took the system a step further by seeding the flow with a hydrogel that turns into a polymer when exposed to light. Then, by shining light patterns on the flow, the scientists can create rigid or flexible structures inside the active fluid. In this case, they show off some of the neat flow patterns they can create. (Video and image credit: G. Pau et al.)

Drying Unaffected by Humidity
Water evaporates faster in dry conditions than in humid ones, but the same isn’t true of paint. Instead, paint’s drying time is largely independent of the day’s humidity. That’s because of paint’s long chains of polymers. As water in the paint evaporates, these polymers are drawn to the surface, forming a viscoelastic layer that hinders evaporation and keeps the drying rate independent up to about 80 percent humidity.

Illustration depicting evaporation of water (left) and evaporation of a polymer solution (right). As water evaporates from the polymer solution, it draws polymers to the surface, where they form a layer that hinders evaporation and makes its rate independent of humidity. The polymer layer explains why evaporation isn’t affected by humidity at longer times, but researchers also saw humidity-independent evaporation early in their experiments. Under a microscope, they discovered a thin gel layer (top image) covering the air-polymer interface. They propose that this fast-forming layer further hinders evaporation. Their findings may be significant for virus-laden respiratory droplets, which also contain polymers. (Image and research credit: M. Huisman et al.; see also J. Salmon et al.; via APS Physics)

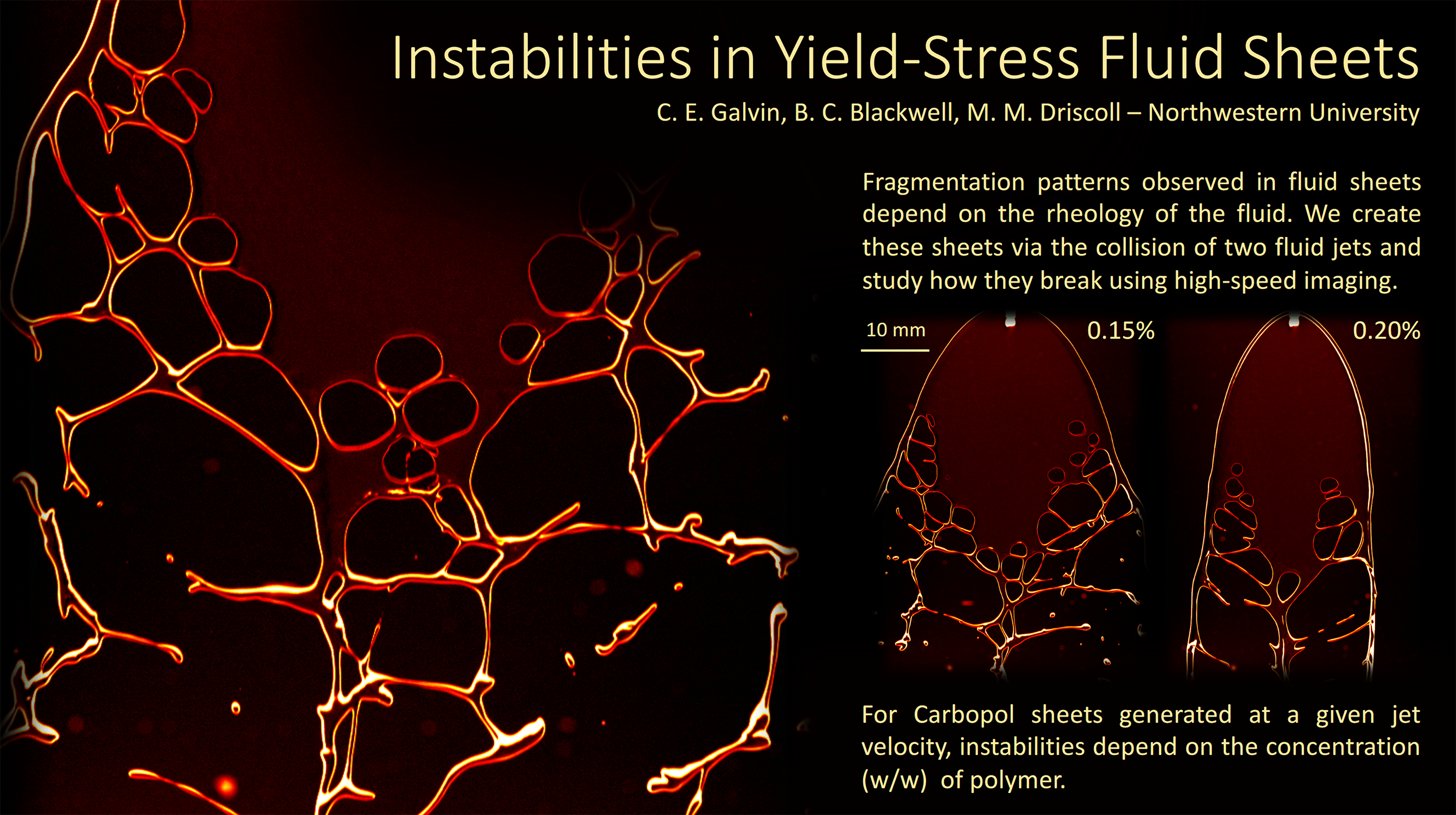
Polymers and Fluid Sheets
Even adding a small amount of polymers to a fluid can drastically change its behavior. Often polymer-doped fluids act more like soft solids, able to hold their shape like your toothpaste does when squeezed onto your toothpaste. Under a little stress, though, the fluids still flow; that’s why your toothpaste gets less viscous as you scrub.
To study the changes polymers make, this research team collides two jets of fluid to create a liquid sheet. Depending on the flow rate and the added polymers, the break-up pattern of the sheet changes. By observing changes in the sheet thickness and the holes that form, they can draw conclusions about what the polymers are doing. (Video credit: C. Galvin et al.)

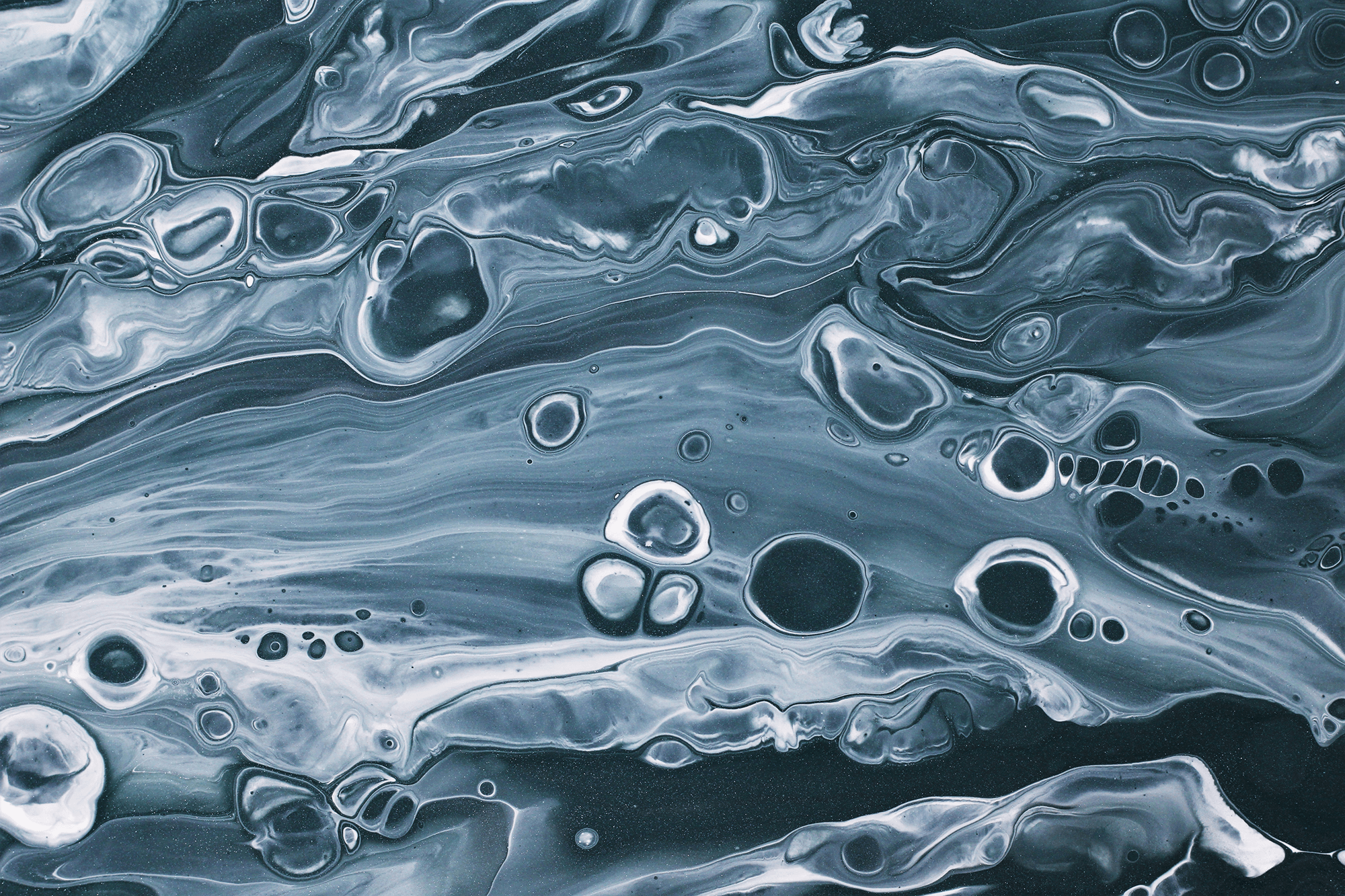
“Keeping Our Sheet Together”
When two liquid jets collide, they form a falling liquid sheet. Here researchers explore how that sheet breaks up when the liquids involved contain polymers. The intact areas of the sheet show as dark red or almost black. The edges of the sheet appear in brighter red and yellow, outlining the holes that form and grow during breakup. The type of breakup observed depends on the concentration of polymer in the liquid. (Image credit: C. Galvin et al.)

Elastic Turbulence
Decades ago, engineers pumping polymer-filled drilling liquids into porous rock noticed sudden and dramatic increases in the viscosity of the liquid. Within the tiny pores of the rock, conventional (i.e., inertial) turbulent flow should be impossible — the Reynolds number is simply too low. Now a new experiment points to the source of the high viscosity: elastic turbulence.
To observe the phenomenon, researchers watched flow in the spaces between glass beads packed into a narrow channel. Videos of flow through one of these pores — roughly 250 microns across — are shown below. When flow rates are low (left), the fluid moves smoothly through the pore, but at higher flow rates (right), chaotic fluctuations emerge, creating the dramatic increase in apparent viscosity. In their analysis, the researchers found that the polymers’ motions generated the flow fluctuations, but most of the viscosity increase was inherent to the fluid’s movement, not to the polymers’ resistance to stretching. (Image credit: top – M. van den Bos, pore flow – Datta Lab; research credit: C. Browne and S. Datta; via Quanta Magazine; submitted by Kam-Yung Soh)

At low flow rates (left), the fluid moves smoothly through the tiny pores, but at higher flow rates (right), the polymers in the flow generate elastic turbulence that greater increases the fluid’s apparent viscosity. 
Dripping With Particles
Adding just a little polymer to a fluid can make it viscoelastic and drastically change how it drips. A pure, viscoelastic fluid (left) necks down to a thin filament thanks to the polymers’ resistance to being stretched. But what happens when you add particles, too?
That’s the focus of this recent study, which adds particles of different sizes to dripping viscoelastic fluids. The researchers found that particles sped up how quickly the filament thinned and formed bead-like droplets. And larger particles (right) made the process even faster than small ones (middle), in experiments where the overall volume fraction of particles within the fluid matched. (Image and research credit: V. Thiévenaz and A. Sauret)

Snapping When Swollen
The Venus flytrap snaps shut on its hapless prey by swelling cells in its leaves with water. Under the added pressure of a fly’s footstep, the leaves’ snapping instability triggers, trapping the insect. Researchers are using similar physics to create jumping and snapping polymer gels, like the one seen below.

To trigger the behavior, researchers soaked their polymer-based gel strips and shells in a solvent of n-hexane, which easily permeated the material and made it swell up. As the solvent evaporates from the swollen gel, the polymer material changes shape, sometimes in smooth bends and sometimes in abrupt snaps. The group was able to harness those snaps to have their materials descend slopes and climb ladders — all without motors, batteries, or external sources of energy. (Image credit: plant – A. Dénes, shell – Y. Kim et al.; research credit: Y. Kim et al.; via Physics World)

Reader Question: Kinetic Sand
An inquiring reader wants to know:
How does kinetic sand work to make it flow like a liquid? Thanks!
– 3 Year Olds EverywhereI confess I don’t have any firsthand experience with Kinetic Sand, but it certainly looks fun. It’s a colorful, moldable sand toy that holds together far better than your typical pile of sand. From what I’ve been able to find, the secret ingredients are a little bit of polydimethylsiloxane (PDMS) — a type of silicon-based polymer — and olive oil, which coats the sand and keeps it from drying out.
PDMS is viscoelastic, which is what gives the Kinetic Sand its unique properties. When a force is applied quickly, the material reacts like a solid, which is why you can mold or cut the sand and have it maintain its shape. But when left alone for awhile under gravity’s influence, the sand will flow like a liquid. This combination of behaviors usually comes down to the polymers in the material. When forces try to stretch these long molecules quickly, they resist; that’s what creates the elasticity of the material. On the other hand, when a force is gradual, the complex molecules have the time to untangle and relax, allowing the material to flow. (Image credit: Kinetic Sand, source)

Storm Eyes and Mushrooms in a Drop
In industry, drying droplets often have many components: a liquid solvent, solid nanoparticles, and dissolved polymers. The concentration of that last component — the polymers — can have a big effect on the way the droplet dries, as seen in the video above.
Without polymers, the droplet dries similarly to a coffee ring stain. But at moderate concentration, we see something very different. The droplet forms an eye in the middle, similar to a hurricane’s, and the edges of the droplet sprout mushroom-shaped plumes that grow and merge with one another along the edge. With even larger polymer concentrations, the mushrooms sweep their way inward, leaving a feathery stain behind. (Video, image, and research credit: J. Zhao et al.)