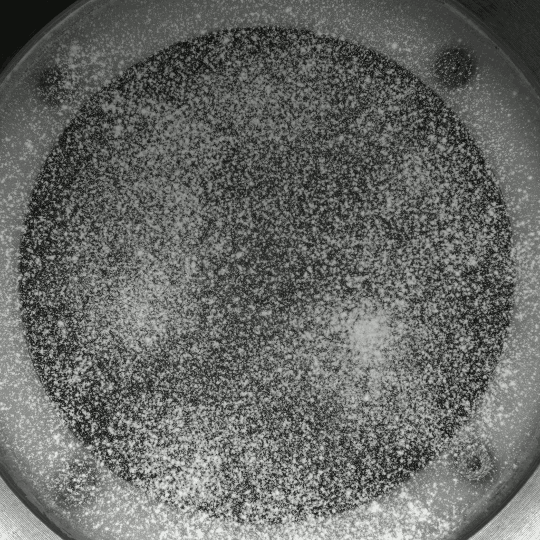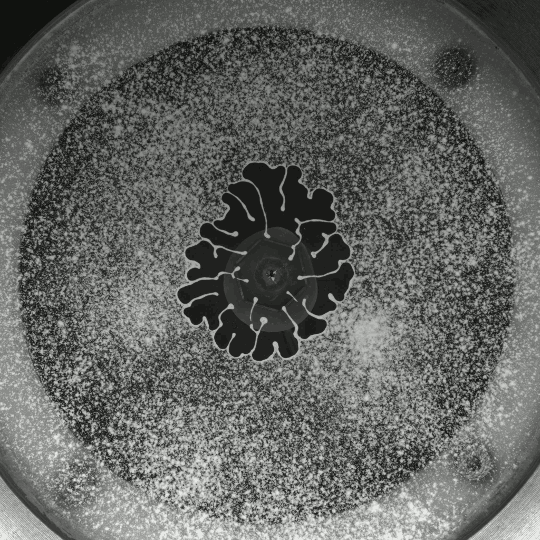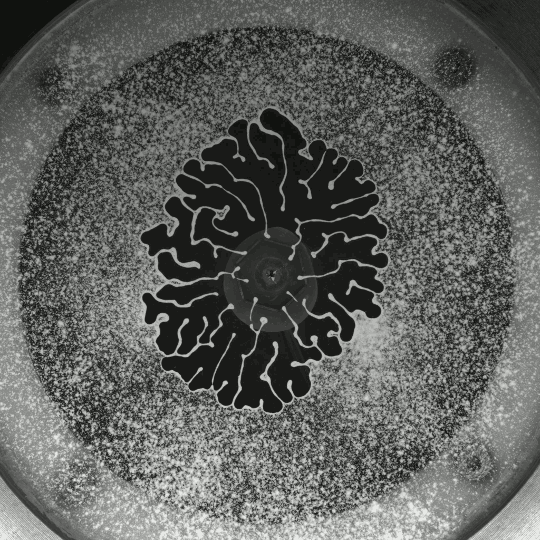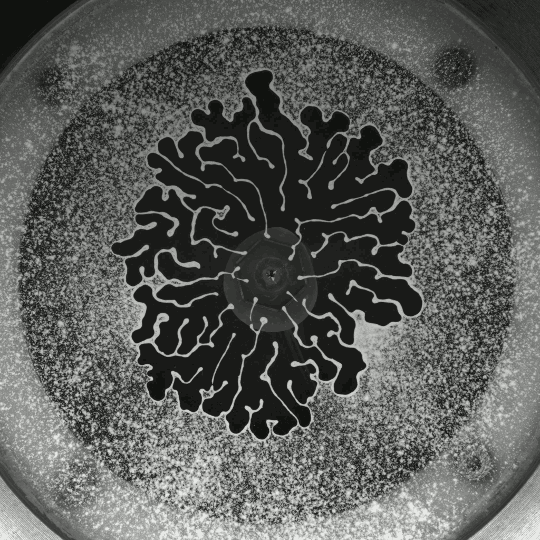
Beautiful colors, subtle flows, and sudden fractals animate Thomas Blanchard’s “Sensations,” which, like his other short films, is entirely CGI-free. It’s a lovely exploration of droplets, liquid lenses, Marangoni effects, and fingering instabilities. (Video and image credit: T. Blanchard)
Tag: fingering instability

Frictional Fingers
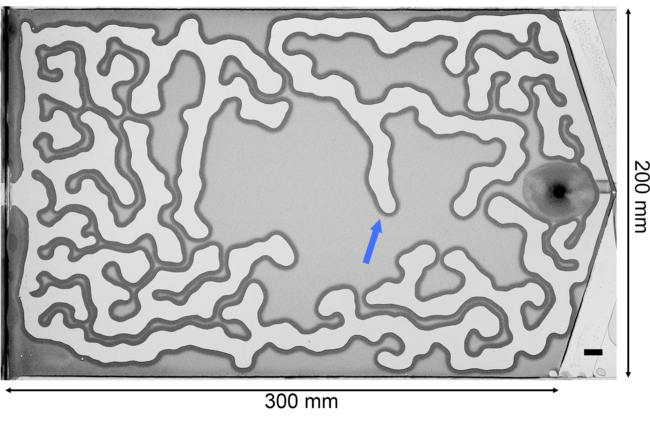
Air pushes into a thin gap filled with water and granular particles in the labyrinth-like image above. The encroaching air pushes grains like a bulldozer’s blade, building up a compacted wall. The invasion continues until the pressure of the air is countered by the combined capillary and frictional forces of the wet grains. Researchers built an analytical model that explains how these frictional fingers form and grow. Unlike Saffman-Taylor fingering patterns, which depend on long-range viscous forces, these patterns depend entirely on short-range forces from surface tension and friction. (Image and research credit: E. Flekkøy et al.)

Growing Metal Fingers
Eutectic gallium-indium alloy is a room-temperature liquid metal with an extremely high surface tension. Normally, that high surface tension would keep it from spreading easily. But once the metal oxidizes, the surface tension drops. When that oxidation is combined with an electric field, the metal spreads into fingers. The higher the voltage, the more complex the fingering patterns. (Image and video credit: K. Hillaire et al.)

“Dendrite Fractals”
In this short film from the Chemical Bouillon team, dark ink drops spread in dendritic fractal patterns after being deposited on an unknown transparent liquid. Although the patterns look similar to those of the Saffman-Taylor instability, I suspect what we see here is actually driven by surface tension and not viscosity.
The authors describe the ink they used as a “special old” “tree ink,” which — putting on my fountain pen aficionado hat — probably means some variety of iron gall ink. These inks draw on chemicals extracted from trees and other plants to create a permanent, waterproof ink. They tend to be highly acidic, which could play a role in the pattern formation seen here. (Video and image credit: Chemical Bouillon)

Pearls On a Puddle
Leave a drop of coffee sitting on a surface and it will leave behind a ring of particulates once the water evaporates. But what happens to a droplet made up of multiple liquids that evaporate differently? That’s the subject of this new study. Researchers mixed a volatile drop (isopropyl alcohol) with a smaller amount of a non-volatile liquid and observed how this changed the droplet’s splash rim and evaporation pattern.
When the surface tension difference between the two liquids was large, the researchers found that the splash formed fingers along its rim (Image 1). The fingers consist almost entirely of the non-volatile component, driven to the outskirts of the drop by Marangoni forces. The dark and light bands you see in the image are interference fringes, which the researchers used to track the film’s thickness.
When the researchers used liquids with similar surface tensions, the droplet rim instead formed pearl-like satellite droplets. Once the volatile liquid evaporated away, the remaining liquid merged into a thick film. (Image and research credit: A. Mouat et al.; via APS Physics; submitted by Kam-Yung Soh)

Coalescence
Simple acts like the coalescence of two droplets sitting on a surface can be beautiful and complex. As the droplets come together, they form a thin neck between them, and the curvature of that surface causes capillary forces that drive fluid into the neck. For two dissimilar droplets, like the ones above, there can be additional forces. Here, the upper drop is pure water, but the lower one has added surfactants, which reduce its surface tension. That difference in surface tension creates a Marangoni flow that tends to pull fluid away from the neck. The result is that full coalescence takes longer. Depending on other factors in this tug-of-war between capillary action and Marangoni flow, the process of coalescence can look very different. In this example, there’s a fingering instability that occurs as the neck spreads. Change the circumstances slightly and the drops may chase each other instead of merging or will merge with a perfectly smooth contact front. (Image and research credit: M. Bruning et al.)

Porous Fingers
If you inject a less viscous fluid, like air, into a narrow gap between two glass plates filled with a more viscous fluid, you’ll get a finger-like instability known as the Saffman-Taylor instability. If you invert the situation – injecting something viscous like water into air – the water will simply expand radially; you’ll get no fingers. But that situation doesn’t hold if there are wettable particles in the air-filled gap. Inject water into a particle-strewn air gap and you get a pattern like the one above. In this case, as the water expands, it collects particles on the meniscus between it and the air. Once the concentration of particles on the meniscus is too high for more particles to fit there, the flow starts to branch into fingers. This creates a greater surface area for interface so that more particles can get swept up as the water expands. (Image and research credit: I. Bihi et al., source)

Fluid Fingers
Differences in viscosity or surface tension between two fluids can lead to finger-like instabilities. Here food dye placed on corn syrup forms narrow tendrils driven by the differing surface tensions of the two liquids. Similar dendritic shapes can be generated by injecting a low viscosity fluid into a high viscosity one (Saffmann-Taylor instability) or by pulling apart glass plates sandwiched around a high viscosity fluid. (Photo credit: T. Gaskill et al.)

The Challenges of Trapping Carbon Dioxide
One way to reduce carbon dioxide in the atmosphere is to pump the CO2 into saline aquifers deep below the surface. Such aquifers are thin but stretch over large areas and are sometimes gently sloping. Since carbon dioxide is relatively buoyant, it may migrate up-slope after injection and potentially leak elsewhere. Dissolving the carbon dioxide into the groundwater helps prevent this undesirable migration. The video above shows a laboratory analog of the fluid instability at the heart of this trap. Imagine the video tilted by a few degrees so it slopes upward toward the right. The initially buoyant carbon dioxide, represented by the dark fluid, rises on the left and moves rightward, up-slope. As the CO2 dissolves into the ambient groundwater, the water becomes denser and fingers of the CO2-rich water drift downward, effectively halting the carbon dioxide’s escape. This is known as convective dissolution. (Video credit: C. MacMinn and R. Juanes)

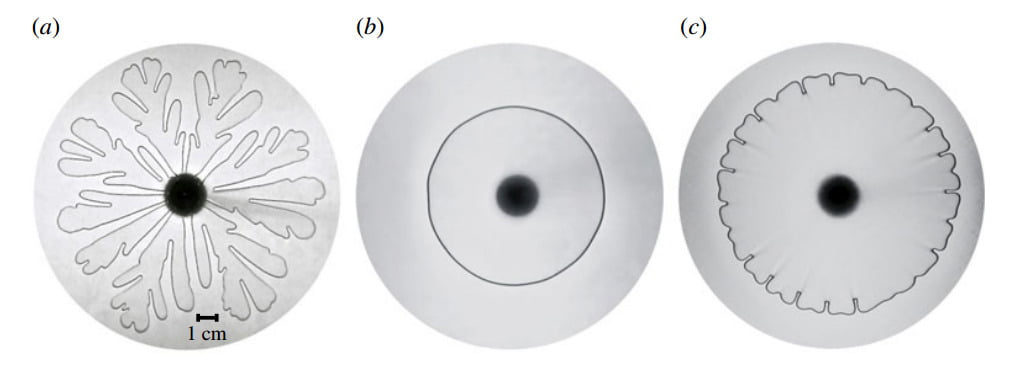
Elastic Walls and Viscous Fingers
The Saffman-Taylor instability, characterized by the branchlike fingers formed when a less viscous fluid is injected into a more viscous one, is typically demonstrated between two rigid walls, as in part (a) of the figure above. But what happens if one of the rigid walls forming the Hele-Shaw cell is replaced with an elastic wall? This is the case for (b) and (c) in the figure. The flexibility of the wall causes the expansion of the air-fluid interface to slow down relative to the rigid wall case and causes the interface to move toward a narrowing fluid-filled gap (as opposed to a constant thickness one). Both of these effects reduce the viscous instability mechanism that drives the fingering instability. With a high enough mass flow rate as in ©, there is still some instability in the interface, but it is dramatically reduced. (Photo credit: D. Pihler-Puzovic et al.)