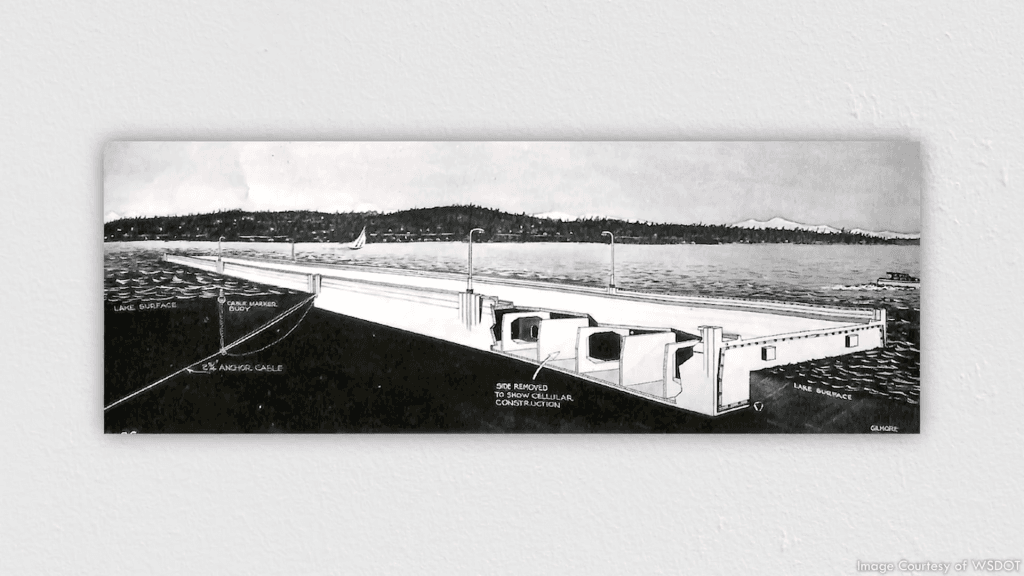
For most of history, floating bridges have been temporary structures, often used by militaries crossing water, but over the course of the twentieth century, engineers learned to build more permanent floating bridges. These structures require very particular conditions–calm waters, minimal ice, and so on–but they can be great options for crossing lakes where the traditional anchoring options for a bridge just don’t exist. In this Practical Engineering video, Grady discusses some of the challenges and innovations of these unusual bridges. (Video and image credit: Practical Engineering)
Tag: buoyancy

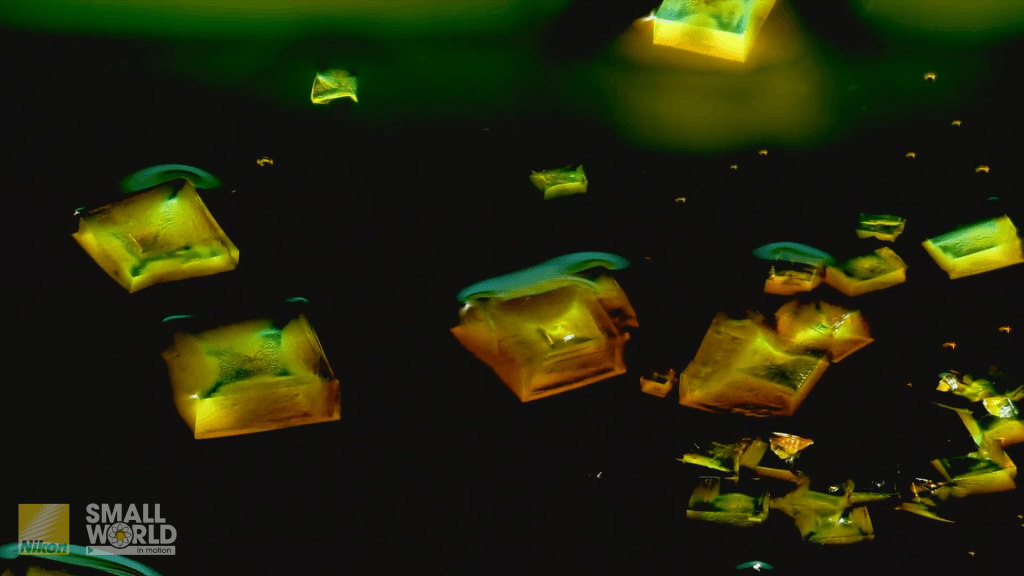
Dissolution and Crystallization
A colorful assortment of salts dissolve and recrystallize in this microscopic timelapse video by retired engineer Jay McClellan. Every step is a gorgeous rainbow of color as the cobalt, copper, and sodium chlorides dissolve, mix, and change. Though we don’t see what’s going on in the water, fluid dynamics are a critical component of both dissolution and crystallization. In the former, concentration gradients change the water’s density, driving buoyant flows. For the latter, crystallization comes out of evaporation, where surface tension often determines where solid particles get left behind. (Video and image credit: J. McClellan; via Colossal)

Dispersing Pollutants via Smokestack
In our industrialized society, pollutants are, to an extent, unavoidable. Even with technologies to drastically reduce the amount of pollutants leaving a factory or plant, some will still get released. It’s up to engineers to make sure that those released spread out enough that their overall concentration does not pose a risk to public health. In this Practical Engineering video, Grady explains some of the physics and engineering considerations that go into this task.
As he demonstrates, taller smokestacks speed up the buoyant exhaust plume (to an extent), which exposes the plume to higher winds, greater turbulence, and, thus, quicker dispersal. But atmospheric conditions and even nearby buildings all affect how a plume spreads. (Image and video credit: Practical Engineering)

Seeking Randomness
Securing information on the Internet requires a lot of random numbers, something computers are not good at creating on their own. This need for random input raises an important philosophical and practical question: what is randomness? How can we be sure that something truly is random, or is it enough for a system to be practically random? Joe explores these questions in this Be Smart video, which shows off how companies use systems — including fluid dynamical ones like lava lamps and wave machines — to generate random numbers for encryption. (Video and image credit: Be Smart)

How Particles Affect Melting Ice
When ice melts in salt water, there’s an upward flow along the ice caused by the difference in density. But most ice in nature is not purely water. What happens when there are particles trapped in the ice? That’s the question this video asks. The answer turns out to be relatively complex, but the researchers do a nice job of stepping viewers through their logic.
Large particles tend to fall off one-by-one, which doesn’t really affect the buoyant upward flow along the ice. In contrast, smaller particles fall downward in a plume that completely overwhelms the buoyant flow. That strong downward flow makes the ice ablate even faster. (Video and image credit: S. Bootsma et al.)

Bubbly Tornadoes Aspin
Rotating flows are full of delightful surprises. Here, the folks at the UCLA SpinLab demonstrate the power a little buoyancy has to liven up a flow. Their backdrop is a spinning tank of water; it’s been spinning long enough that it’s in what’s known as solid body rotation, meaning that the water in the tank moves as if it’s one big spinning object. To demonstrate this, they drop some plastic tracers into the water. These just drop to the floor of the tank without fluttering, showing that there’s no swirling going on in the tank. Then they add Alka-Seltzer tablets.
As the tablets dissolve, they release a stream of bubbles, which, thank to buoyancy, rise. As the bubbles rise, they drag the surrounding water with them. That motion, in turn, pulls water in from the surroundings to replace what’s moving upward. That incoming water has trace amounts of vorticity (largely due to the influence of friction near the tank’s bottom). As that vorticity moves inward, it speeds up to conserve angular momentum. This is, as the video notes, the same as a figure skater’s spin speeding up when she pulls in her arms. The result: a beautiful, spiraling bubble-filled vortex. (Video and image credit: UCLA SpinLab)


“My Own Galaxy”
Fungal spores sketch out minute air currents in this shortlisted photograph by Avilash Ghosh. The moth atop a mushroom appears to admire the celestial view. In the largely still air near the forest floor, mushrooms use evaporation and buoyancy to generate air flows capable of lifting their spores high enough to catch a stray breeze. (Image credit: A. Ghosh/CUPOTY; via Colossal)

How Cooling Towers Work
Power plants (and other industrial settings) often need to cool water to control plant temperatures. This usually requires cooling towers like the iconic curved towers seen at nuclear power plants. Towers like these use little to no moving parts — instead relying cleverly on heat transfer, buoyancy, and thermodynamics — to move and cool massive amounts of water. Grady breaks them down in terms of operation, structural engineering, and fluid/thermal dynamics in this Practical Engineering video. Grady’s videos are always great, but I especially love how this one tackles a highly visible piece of infrastructure from multiple engineering perspectives. (Video and image credit: Practical Engineering)

Billowing Ouzo
Pour the Greek liquor ouzo into water, and your glass will billow with a milky, white cloud, formed from tiny oil droplets. The drink’s unusual dynamics come from the interactions of three ingredients: water, oil, and ethanol. Ethanol is able to dissolve in both water and oil, but water and oil themselves do not mix.
In this video, researchers explore the turbulent effects of pouring ouzo into water. In particular, pouring from the top creates a fountain-like effect, due to a tug-of-war between the ouzo’s momentum and its buoyancy. Momentum wants the ouzo to push down into the water, and buoyancy tries to lift it back up. For an extra neat effect, they also show what happens when the ouzo is confined to a 2D plane and what happens when momentum and buoyancy act together instead of oppositely. (Image and video credit: Y. Lee et al.)

Toying With Density and Miscibility
Steve Mould opens this video with a classic physics toy that uses materials of different densities as a brainteaser. Two transparent, immiscible liquids fill the container, along with beads of a couple different densities. When you shake the toy, the liquids emulsify, creating a layer with an intermediate density. As the two liquids separate, the emulsified middle layer disappears, causing the beads (which have densities between that of the two original liquids) to come together.
The rest of the video describes the challenges of expanding this set-up into three immiscible liquids and four sets of beads. Along the way, Steve had to contend with issues of miscibility, refractive index, and even chemical solvents. It’s amazing, sometimes, what it takes to make a seemingly simple idea into reality. (Video and image credit: S. Mould)