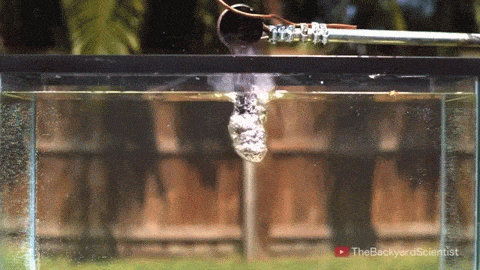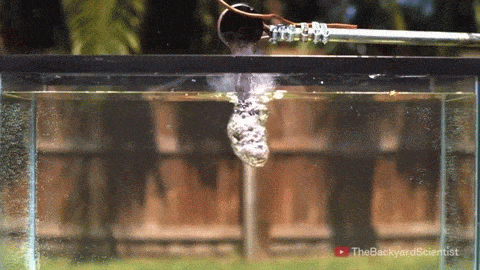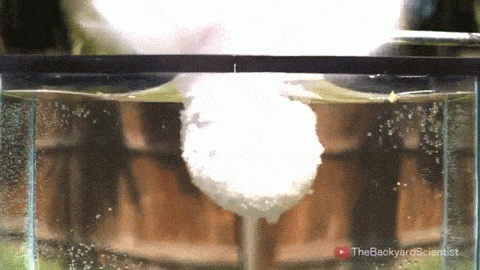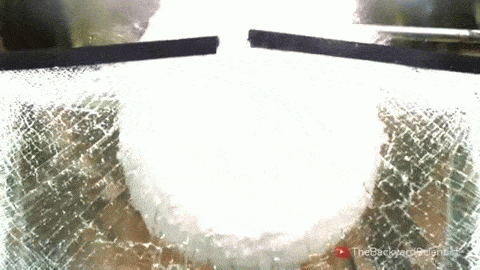
In his latest video, The Backyard Scientist explores what happens when molten salt (sodium chloride) gets poured into water. As you can see, the results are quite dramatic! He demonstrates pretty convincingly that the effect is physical – not chemical. The extreme difference in temperature between the liquid water (< 100 degrees Celsius) and the molten salt (> 800 degrees Celsius) causes the water to instantly vaporize due to the Leidenfrost effect. This vapor layer protects the liquid water from the molten salt – until it doesn’t. When some driving force causes a drop of water to touch the salt without that protective vapor layer, the extreme temperature difference superheats the water, causing it to expand violently, which drives more water into salt and feeds the explosion.
But why don’t the other molten salts he tests explode? Sodium carbonate, the third salt he tests, has a melting point of 851 degrees Celsius, 50 degrees hotter than sodium chloride. Yet for that test, the Leidenfrost effect prevents any contact between the two liquids. The key in this case, I hypothesize, is not simply the temperature difference between the water and salt, but the difference in fluid properties between sodium chloride and sodium carbonate. The breakdown of the vapor layer and subsequent contact between the water and the molten salt depends in part on instabilities in the fluids. A cavity where instabilities can grow more easily is one where the Leidenfrost effect is less likely to protect and separate the two fluids. And, in fact, it turns out that the surface tension of molten sodium chloride is significantly lower than that of molten sodium carbonate! A lower surface tension value means that the molten sodium chloride breaks into droplets more easily and its vapor cavity will respond more strongly to fluid instabilities, making it more likely to come in contact with liquid water and, thus, cause explosions. (Image/video credit: The Backyard Scientist; submitted by Simon H)