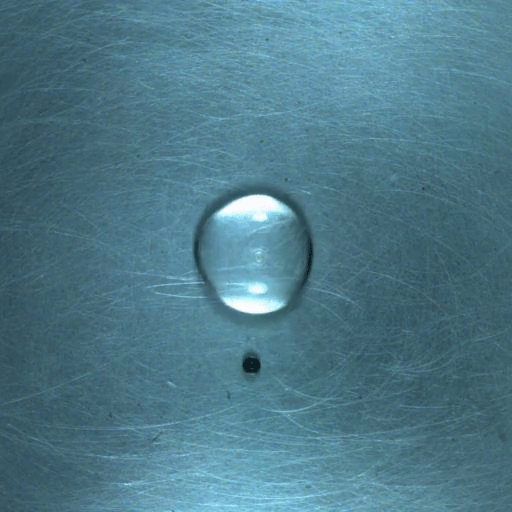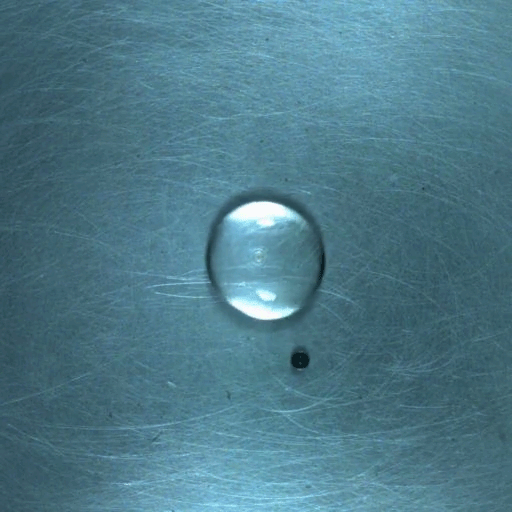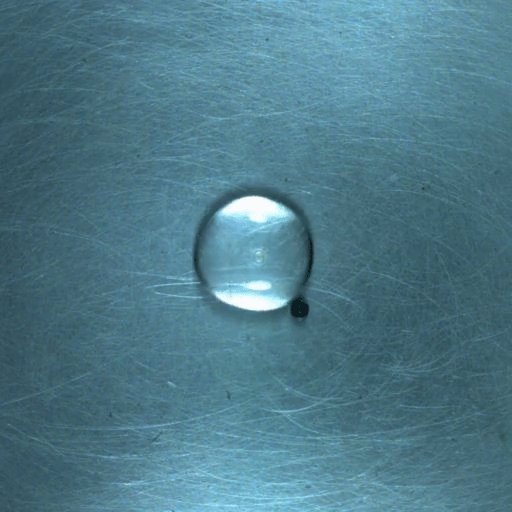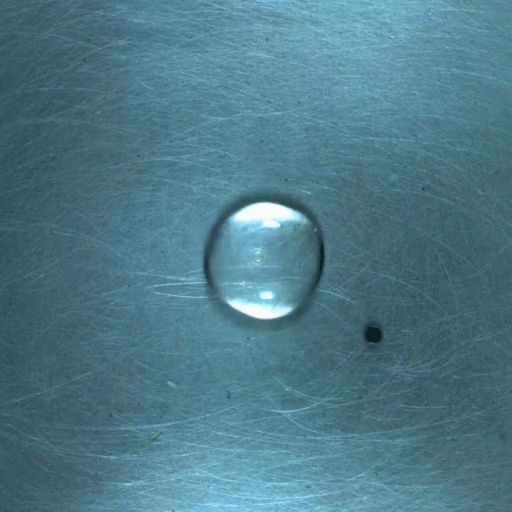
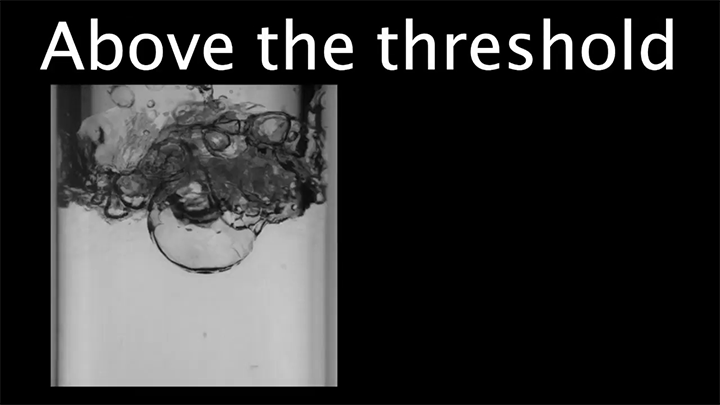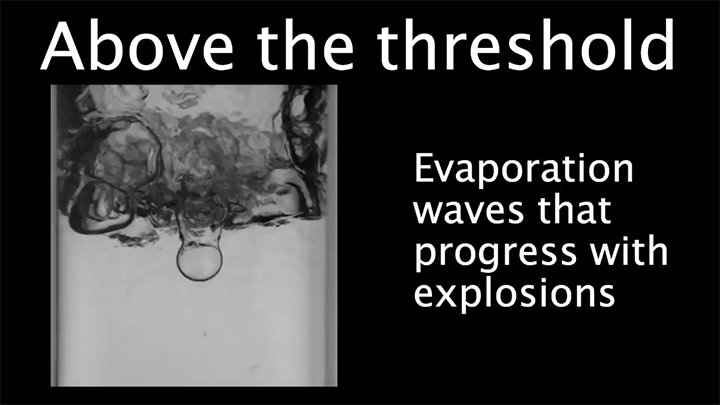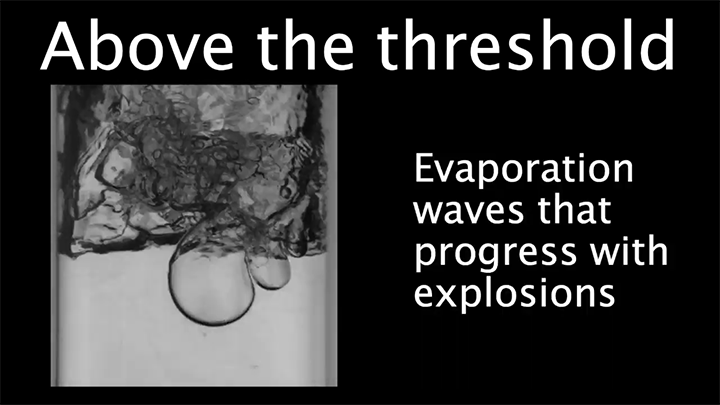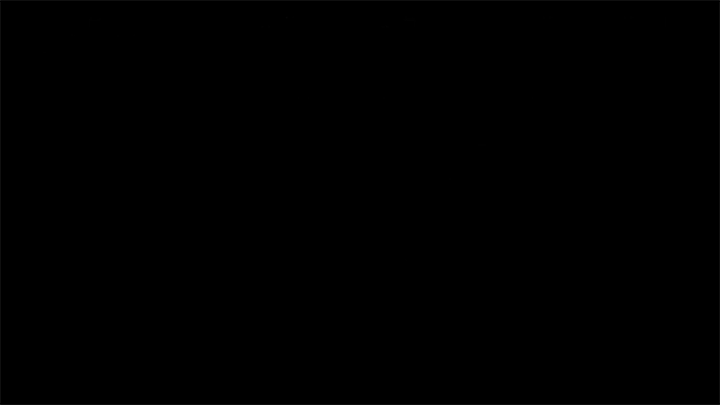
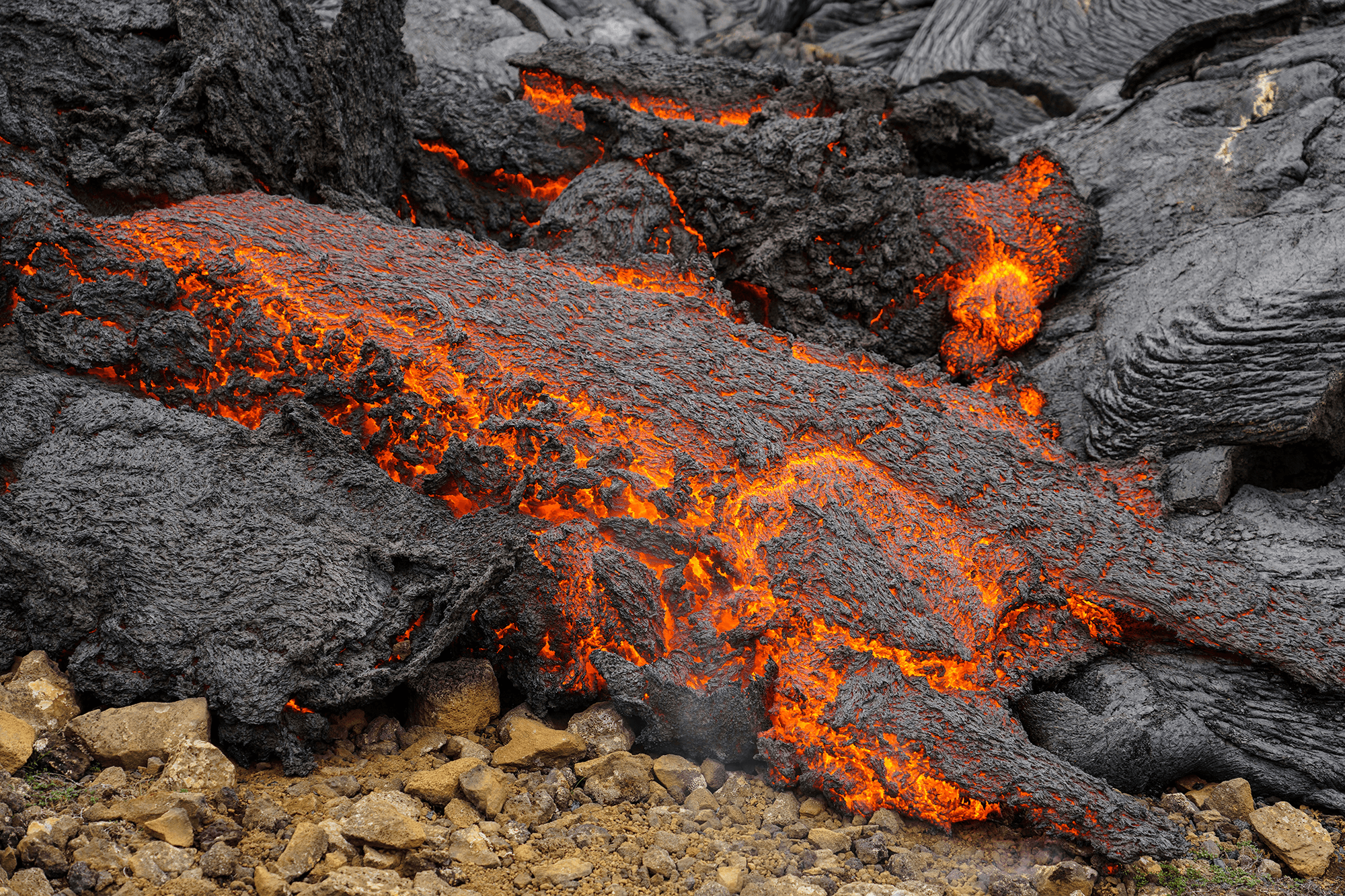Drop water on a surface much hotter than its boiling point, and the liquid will bead up and skitter over the surface, levitated on a cushion of its own vapor. In addition to making the drop hypermobile, this vapor layer insulates it from the heat of the surface, allowing it to survive longer than it would at lower temperatures. Known as the Leidenfrost effect, this phenomenon can show up in lava flows, as well.
Pillow lava is a smooth, bulbous rock formed when lava breaks out underwater. The exiting lava is incandescent and, therefore, incredibly hot — hot enough to vaporize a layer of water surrounding it. The lava can continue to expand until it cools too much to sustain the vapor layer. An elastic skin builds up over the cooling lava. Eventually, a new pillow will bud off, possibly due to a surge in the lava flow or a weak point in the developing skin. (Image credit: J. de Gier; research credit: A. Mills; via LeidenForce)