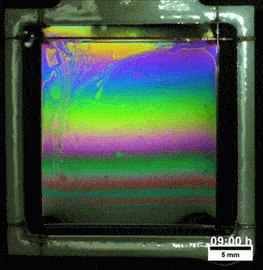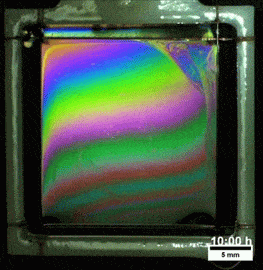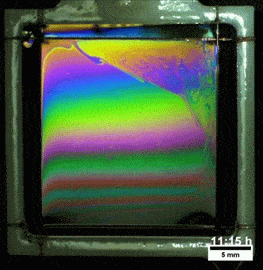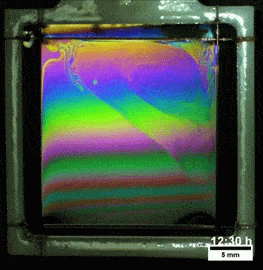
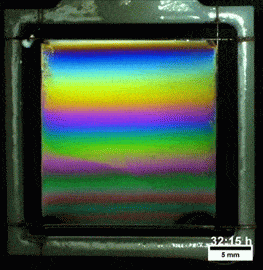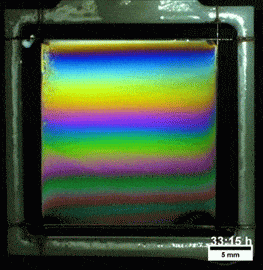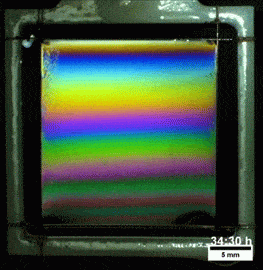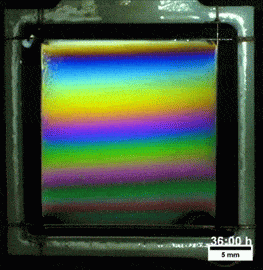
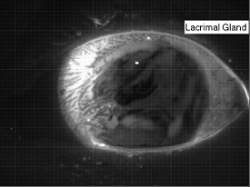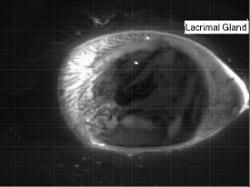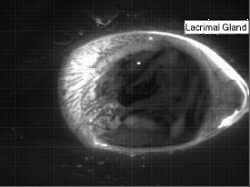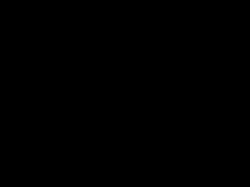
Liquids don’t typically survive very long as thin films. If you try to make one from water, gravity drains it away immediately. (Not so in space.) To make a liquid film stick around, we add surfactants like soap. These extra molecules congregate at the surface of the film and provide a stabilizing force to oppose gravity’s drainage. Exactly what that stabilizing force is depends on the surfactant.
Surfactants that are insoluble are often quite viscous. These molecules distribute themselves across the interface and then they stay. They resist both gravity or even just moving thanks to their high viscosity. That produces a soap film pattern like the one on the right – symmetric and slow to change.
Other surfactants may be soluble in the film and have no appreciable viscosity themselves. These surfactants constantly move and shift on the interface as surface tension variations occur. When weak spots form, the surfactant molecules shift, via the Marangoni effect, to stabilize the film. This creates a film pattern like the familiar one on the right, with an ever-shifting palette of colors. (Image and research credit: S. Bhamla et al., source; submission by S. Bhamla)