Summers in the Barents Sea — a shallow region off the northern coasts of Norway and Russia — trigger phytoplankton blooms like the one in this satellite image. The blue shade of the bloom suggests the work of coccolithophores, a type of plankton armored in white calcium carbonate. This type of plankton thrives in the warm, stratified waters of the late summer. Earlier in the year, the water tends to be nutrient-rich and well-mixed, conditions which favor diatom plankton species instead. Their blooms appear greener in satellite images. (Image credit: W. Liang; via NASA Earth Observatory)
Tag: stratification

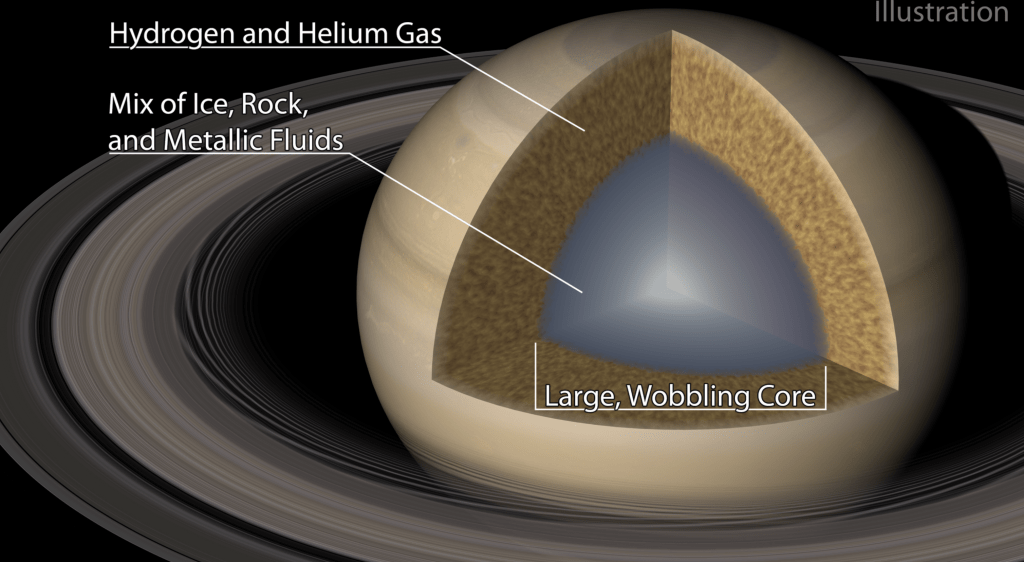
Probing Saturn’s Interior
Saturn’s rings are one of the most iconic sights in our solar system, and scientists are using them to learn more about the planet they surround. Until recently, scientists believed that gas giants like Saturn and Jupiter have dense, rocky cores buried beneath their gassy atmospheres. But a new study of Saturn’s rings suggests that Saturn’s core is far larger and more fluid than assumed.
When the interior of Saturn wobbles, it causes gravitational shifts that affect the material making up its rings. By studying disturbances in the ring system — a technique known as ring seismology — researchers can deduce what motions took place inside the planet to cause the changes in the rings.
Using data from the Cassini spacecraft, the authors determined that Saturn’s core likely spreads to nearly 60% of its radius, and, rather than being dense and rocky, the core is a relatively fluid mixture of ice, rock, and metallic fluids. The core diffuses gradually into the gaseous atmosphere, and it’s stably stratified against convection, so its wobbles are quite small for the planet’s size. (Image credit: rings – NASA; illustration – Caltech/R. Hurt; research credit: C. Mankovich and J. Fuller; via Gizmodo)


Density Drift
This colorful photo shows three fluids — oil, water, and dish soap — illuminated by the rainbow reflection of a CD. The differing densities of each fluid creates a stratification with water sandwiched between dish soap on the bottom and oil on the top. Because the dish soap is miscible in water, it leaves a smudgy blur against the background, whereas the immiscible oil creates bubble-like lenses at the top. (Image credit: R. Rodriguez)

The Undisturbed Waters of Lake Kivu
Deep in Africa lies one of the world’s strangest lakes. Lake Kivu, over 450 meters in depth, is so stratified that its layers never mix. The upper portion of Lake Kivu consists of less-dense fresh water, which sits upon deeper layers of saltier water full of dissolved carbon dioxide and methane pumped into the lake by volcanic activity.
The lake’s lack of convection means that this deep water simply stays put for thousands of years as it collects gases that remain dissolved only thanks to the immense pressure of the water above. Should that deep water be disturbed — by an earthquake, climate changes, or simply oversaturation — the resulting eruption of carbon dioxide could be deadly for the millions of people living nearby. A similar eruption at smaller Lake Nyos in 1986 asphyxiated about 1,800 people.
Fortunately, Lake Kivu is well-monitored, so such an upwelling should not catch observers off-guard. Learn more about Lake Kivu’s oddities over at Knowable. (Image and research credit: D. Bouffard and A. Wüest, via Knowable Magazine; submitted by Kam-Yung Soh)

The Magic* Cork
*Spoiler alert: it’s not magic. It’s science!
Just what makes this dropped cork float beneath the surface? Just like a normal cork, it’s buoyancy! But this seemingly straightforward video is hiding a few key elements. Firstly, the cork has been modified; it has a metal sphere inside it so that its effective density is higher than that of water.
Secondly, that liquid is not pure water; notice the hazy swirls near the bottom of the flask when the cork drops in? This is tap water that’s had a layer of salt dissolving in the bottom of it for the last day. That creates a density gradient with denser, salty water at the bottom and lighter, fresh water at the top. In fluid dynamics, we’d say the fluid is stably stratified; “stratified” meaning that there are distinct layers (strata) of different density and “stably” because the heavier ones are at the bottom.
When the cork is dropped in, it settles at the fluid layer that matches its density. Because the surrounding fluid is stably stratified, poking the cork makes it bounce slightly but return to its initial height. Our atmosphere behaves just like this when it’s stably stratified. If you displace a parcel of air, it will oscillate up and down before settling back to equilibrium. In fact, the cork and the air even bounce at the same frequency! (Video and submission credit: F. Croccolo)

Self-Assembly Under Stratification
Sometimes mistakes lead to great discoveries. After leaving a failed outreach demo overnight, researchers discovered a new mechanism for self-assembling particles. In the initial set-up, a layer of fresh water is poured atop a layer of denser, saltier water. This creates what’s known as a stably stratified fluid, with progressively denser mixtures of salt water as one moves downward. If you pour in particles of an intermediate density (heavier than fresh water and lighter than salt water), they’ll form a layer at one height, and, if you wait overnight, those particles will slowly form a disk-like raft.

This self-assembly is driven by fluid dynamics — not by any attraction between the particles. Because the particles are unable to absorb salt, their boundaries distort the concentration gradients in the surrounding fluid. This generates subtle currents at the particle boundaries, like in the picture above, where flow moves toward the particle at the equator and away at the poles. Larger particle clusters generate stronger flows, allowing them to attract even more particles.
Although the speeds involved are quite slow, this mechanism may play an important role in nature, where stratified flows are common. The researchers speculate, for example, that the effect could be important in the clustering of microplastics in the ocean. (Image and research credit: R. Camassa et al.; see also R. McLaughlin; submitted by Kam-Yung Soh)

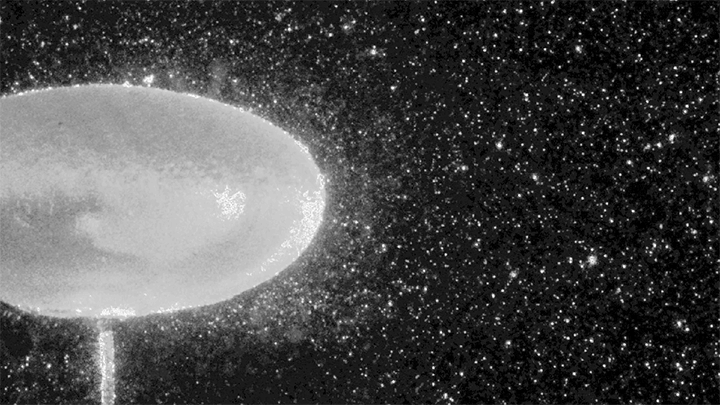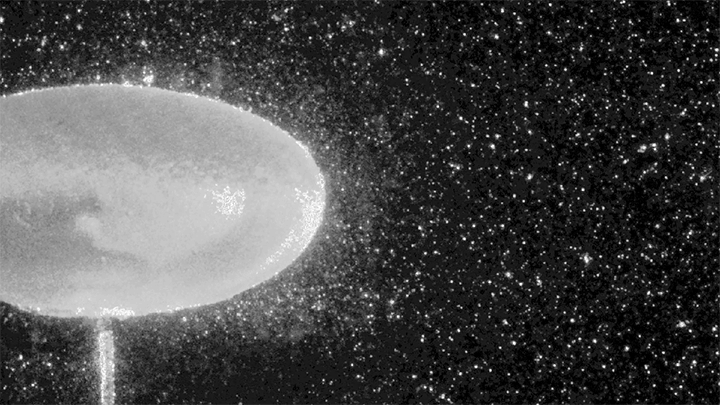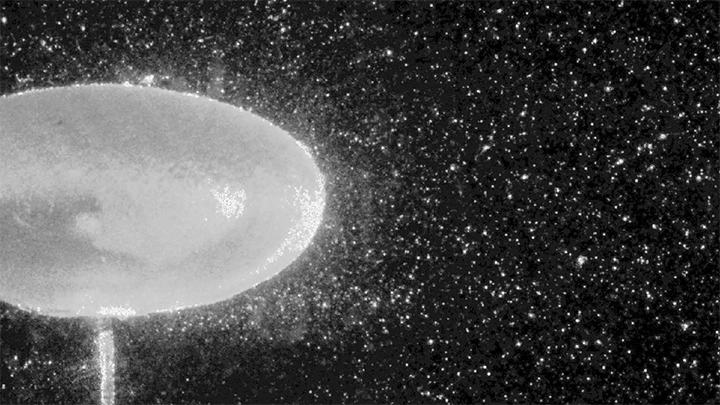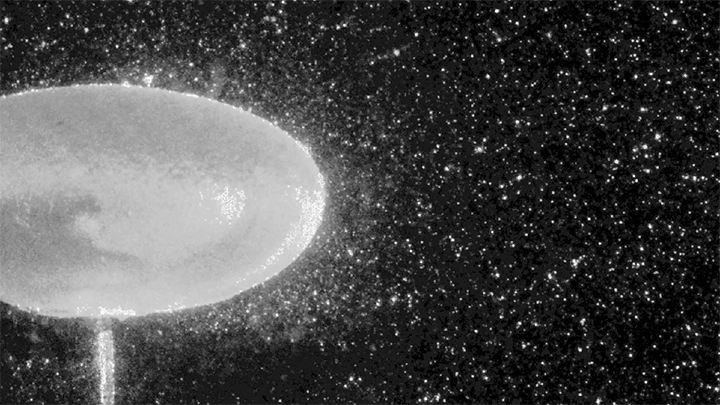
The Bouncing Drop
For a droplet to bounce, we expect it to hit a wall or a sharp interface of some kind. But in a new study, researchers demonstrate a droplet that bounces with neither. Shown above is an oil droplet sinking through a stratified mixture of ethanol (toward the top) and water (toward the bottom). Because the oil is heavier than ethanol, it initially sinks, dragging some of the ethanol with it as it falls. Over time, some of that ethanol rises again, forming what’s known as a buoyant jet.
Simultaneously, the gradient of ethanol to water between the top and bottom of the drop creates an imbalance in surface tension. The ethanol near the top of the drop has a lower surface tension than the water at the bottom. This creates a downward Marangoni flow along the drop interface.
The bounce itself happens quickly after a long, slow sinking period. As the drop’s sinking slows, the buoyant jet weakens until it disappears completely. At the same time, the downward Marangoni flow pulls fresh ethanol-rich fluid toward the top of the drop. That increases the surface tension difference and strengthens the Marangoni flow, creating a positive feedback loop. In less than a second, the Marangoni flow increases by two orders of magnitude, pulling so hard that the drop shoots upward.
That resets the cycle by weakening the Marangoni flow and strengthening the buoyant jet. The droplet can continue bouncing for about 30 minutes until the concentration gradient is so well-mixed that the cycle can’t continue. (Image and research credit: Y. Li et al.; via APS Physics; submitted by Kam-Yung Soh)

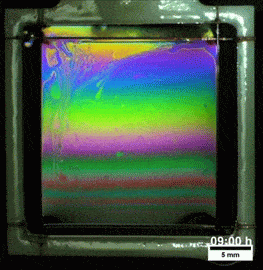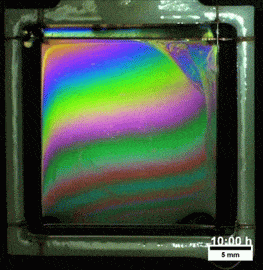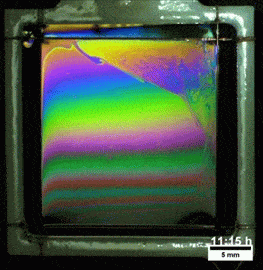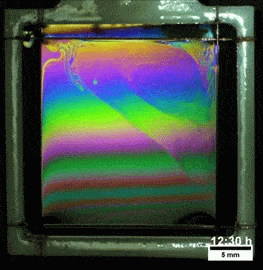
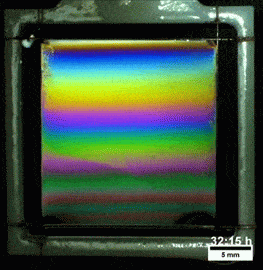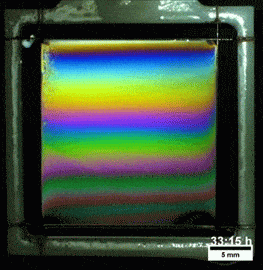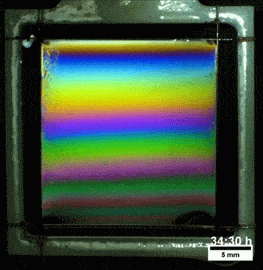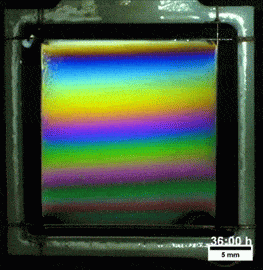
Melted Polymers

What you see here, despite appearances, is not a soap film. On the contrary, this is a thin vertical film made up of melted polymers. Like a soap film, it is extremely thin, varying from a few nanometers at its thinnest to several hundred nanometers at the thickest point. But unlike a freestanding soap film, this polymer film can last for more than a day before the film breaks. Researchers attribute the long life of the films to structural forces inside the fluid.
They observed that the films remain highly stratified, varying smoothly in thickness from their thinnest point at the top to the thickest point at the bottom. They hypothesize that the geometry of the film preferentially traps the polymer’s molecules in preferred orientations, which reinforces the stratification and helps stabilize the film. For more, check out the research paper. (Image credit: T. Gaillard et. al., source; via KeSimpulan)

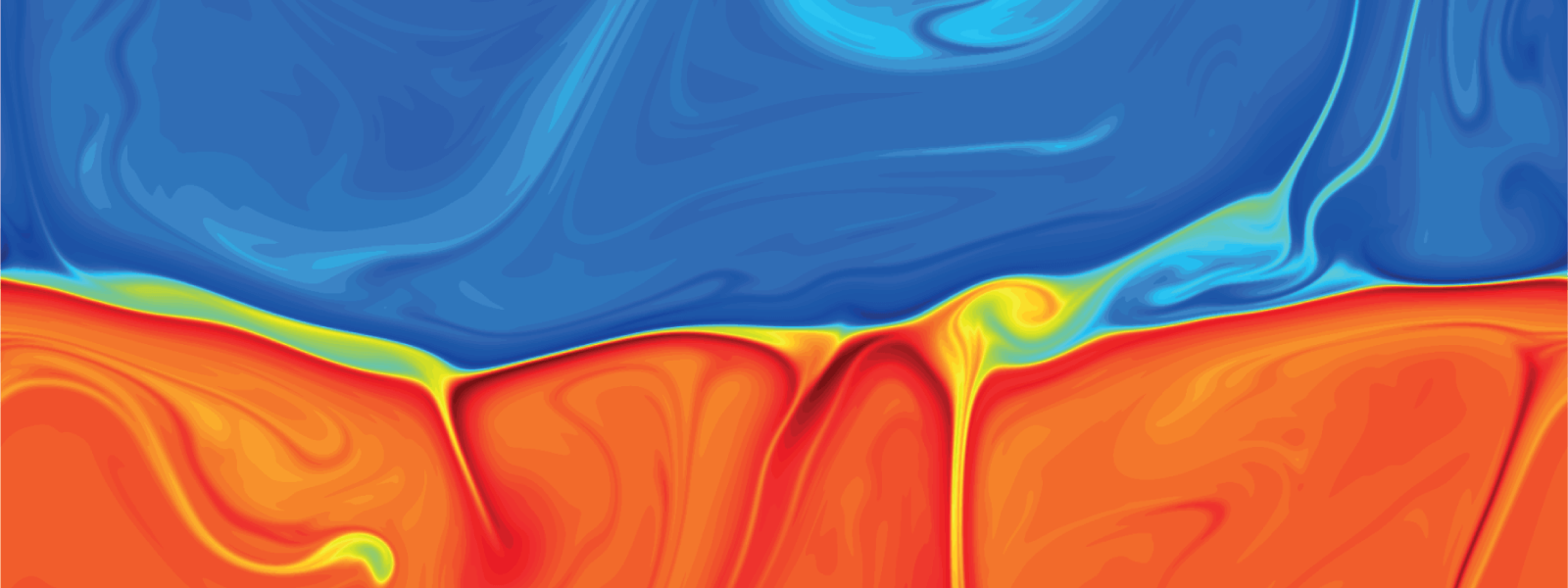
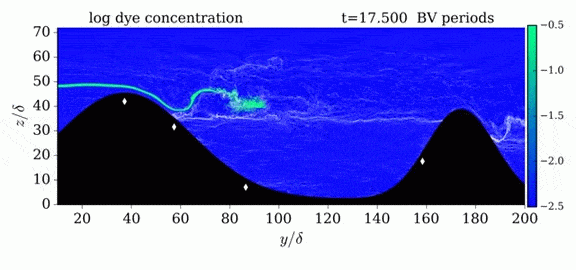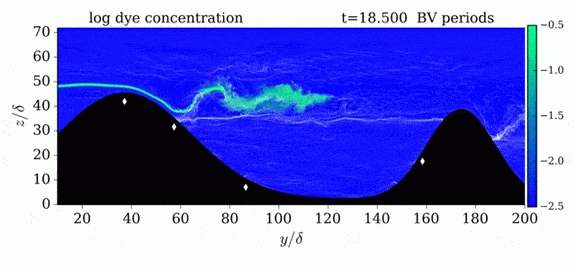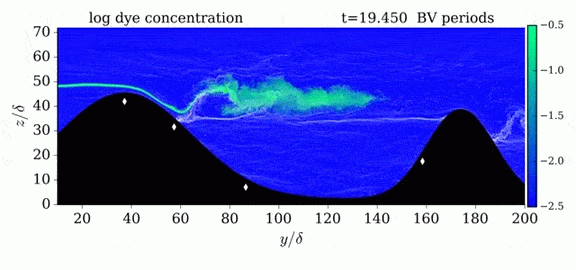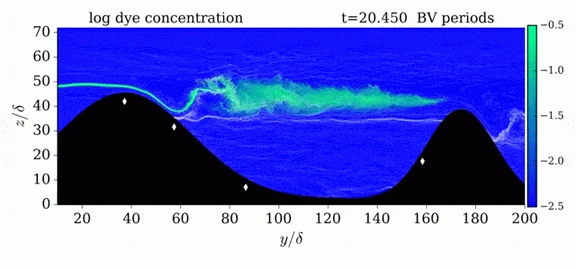
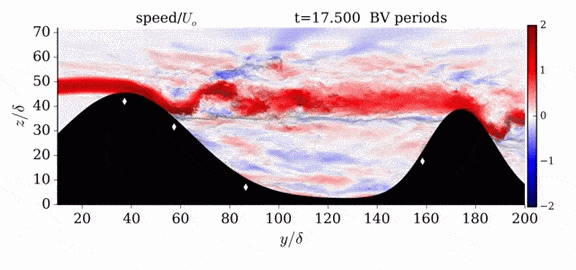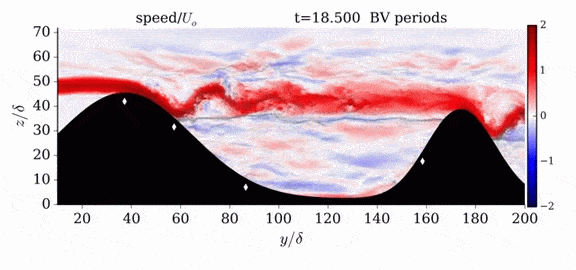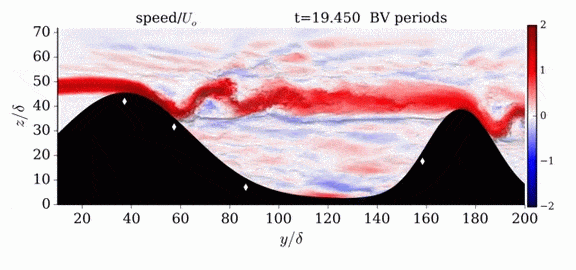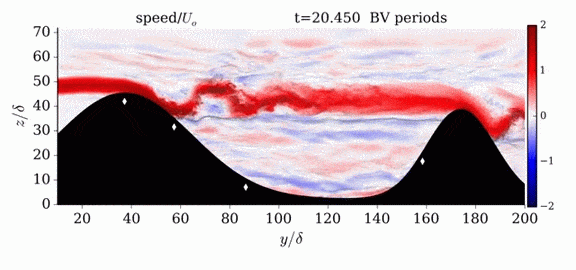
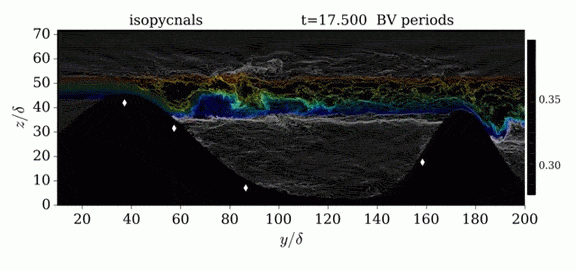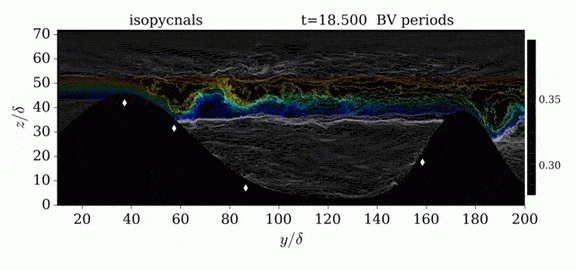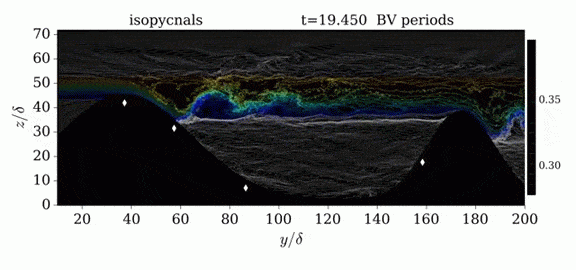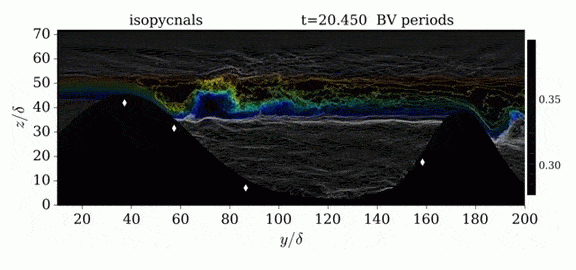
Jumps in Stratified Flows
One of the factors that complicates geophysical flows is that both the atmosphere and the ocean are stratified fluids with many stacked layers of differing densities. These variations in density can generate instabilities, trap rising or sinking fluids, and transmit waves. The animations above show flow over two ridges with dye visualization (top), velocity (middle), and contours of density (bottom). The upstream influence of the left ridge creates a smooth, focused flow that quickly becomes turbulent after the crest. The jet rebounds as a turbulent hydraulic jump before slowing again upstream of the second ridge. Like the first ridge, the second ridge also generates a hydraulic jump on the lee side. Clearly both stratification and the local topography play a big role in how air moves over and between the ridges. If prevailing winds favor these kinds of flows, it can help generate local microclimates. (Image credit and submission: K. Winters, source videos)

Dead Water
Sailors have long known about the “dead water” phenomenon, which can bring ships to a near-standstill, but it was only within the last century that an explanation for the behavior was found. The underlying cause is a stratification of fluids of different densities. As seen in the video above, when a boat moves by exerting a constant force, such as with propellers, it generates an internal wave along the interface between two density layers in the water. As the wave grows in amplitude, it speeds up, chasing and eventually breaking against the boat. The energy that drives the internal wave’s growth comes from the energy the boat expends for propulsion; the larger and closer the wave gets, the slower the boat goes because its energy is sapped by the wave. In the ocean, particularly near sources of freshwater run-off, like melting glaciers, the water can be extremely stratified, with many layers of different salinity and density. The end of the video simulates this with a three-fluid demonstration in which the boat’s motion generates internal waves across multiple density interfaces. (Video credit: M. Mercier et al.)