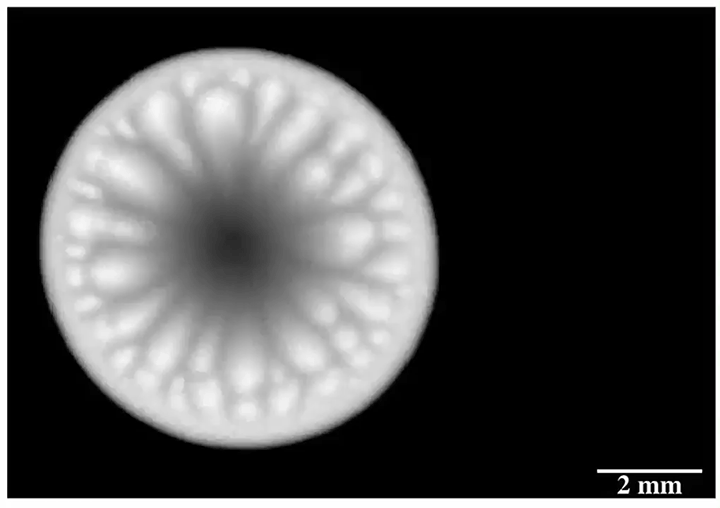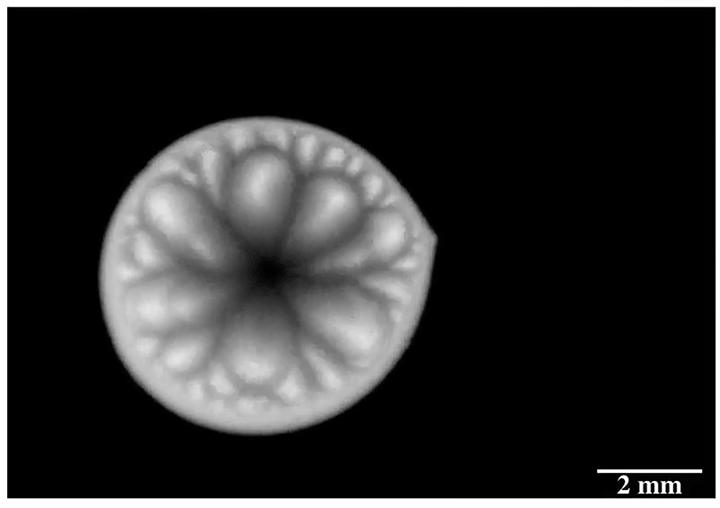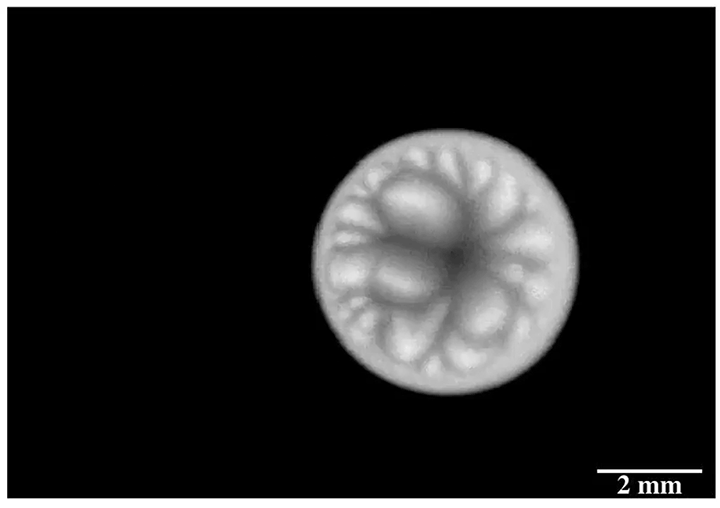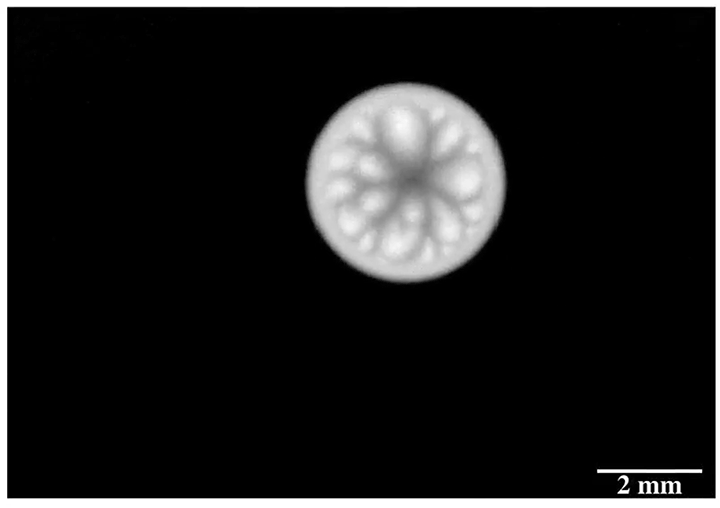
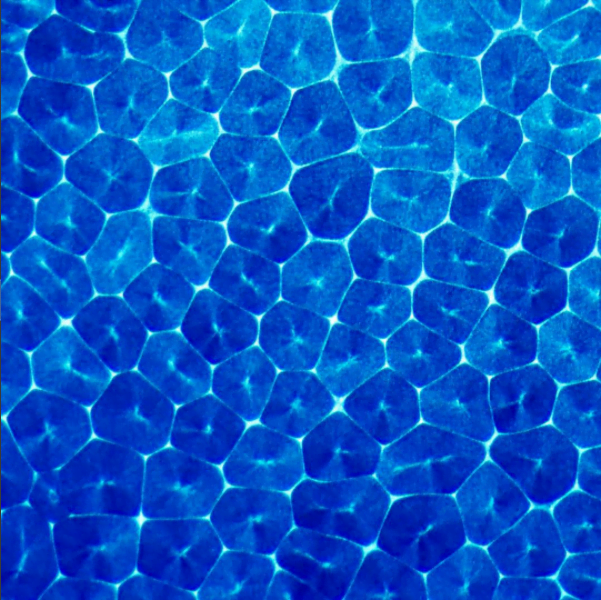
Drip some ethanol on a hot surface, and you’d expect it to spread into a thin layer and evaporate. But that doesn’t always happen, and a recent study looks at why.
Ethanol is what’s known as a volatile liquid, meaning that it evaporates easily at room temperatures, well below its boiling point. When dropped on a uniformly heated surface above 45 degrees Celsius, the drop contracted into a hemisphere and then began to wander randomly across the surface. Researchers trained an infrared camera on the drop from below (above image), and found an unsteady, roiling motion inside the drop. These asymmetric flows, they concluded, drive the drop’s erratic self-propulsion. They suspect the mechanism may explain why some ink droplets wind up in the wrong place on a page during ink-jet printing. (Image and research credit: P. Kant et al.; via APS Physics)