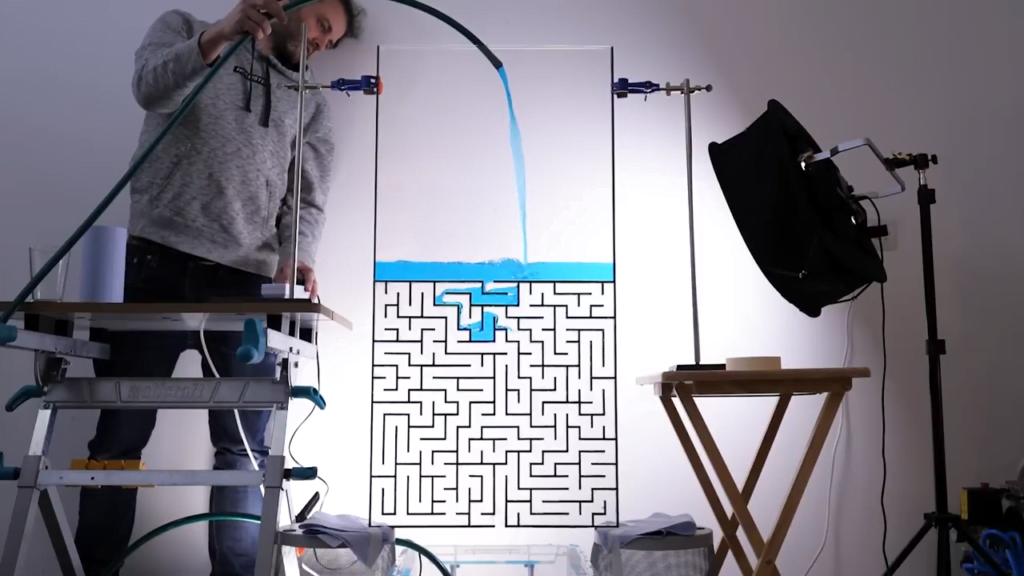
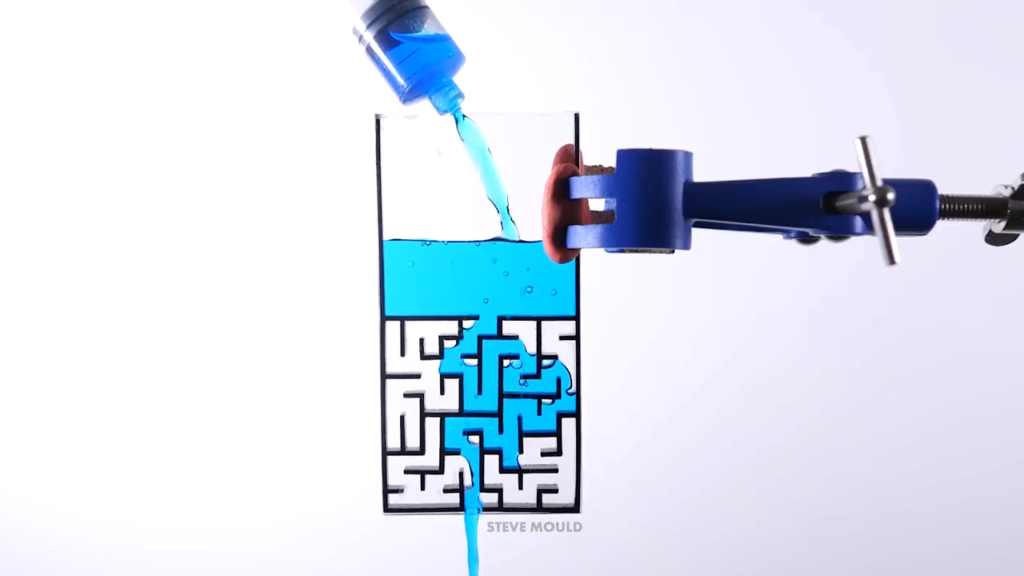
Inspired by a simulation, Steve Mould asks a great question in this video: can water solve a maze? Yes — with some caveats. Steve makes two different maze patterns — a simple and a complex path — in two different sizes. With the small, simple-path version, the water immediately follows the correct path without taking any wrong turns. What keeps it on the right path seems to be a combination of air pressure and surface tension. In the dead-end passages, the air has nowhere to go in order to allow the water in. So the pressure of the trapped air and the narrowness of the passages (which allows surface tension to help hold the water in place) keeps the water out of the false paths.
With the larger mazes, the water is able to take some false turns as it seeks the lowest possible path. But after awhile the incorrect region fills and the water takes the next lowest path available, which eventually leads it to the outlet.
Toward the end of the video, Steve notes that the large mazes sometimes stop flowing, even though water is still in the reservoir. I’ll quibble slightly here with his explanation, though; I don’t think surface tension is playing as much of a role in this stoppage as friction. The water is basically being driven through a long, narrow pipe, which means quite a lot of friction between it and the walls. Just as you need a certain driving pressure to keep water in a pipe flowing, the maze needs a high enough driving pressure to keep the water going. The point at which drainage stops is the point where the upstream pressure (caused by the depth of the reservoir above the maze) is equal to the pressure lost due to friction in the pipe. All in all, it’s a very cool experiment and a video well-worth watching! (Video and image credit: S. Mould)