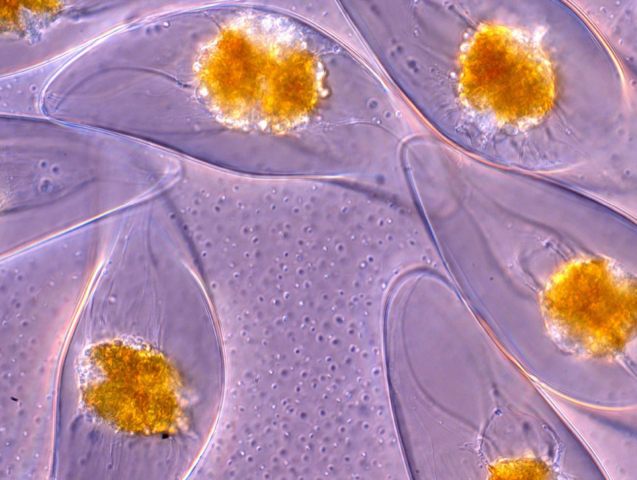
Some single-celled organisms, like dinoflagellates, light up when disturbed. This bioluminescence is considered a defense mechanism, triggered by threats to the organism. Now researchers are quantifying just what it takes to light up a single dinoflagellate.
Dinoflagellates respond both to stress caused by the fluid flow around them and to mechanical deformation — in other words, getting poked. Both methods involve bending and stretching the dinoflagellate’s cell wall, which stretches calcium-ion channels connected to bioluminescence. The researchers found that the intensity of the light produced depended both on the amount and speed of cell wall deformation.
The model built from their observations should help scientists better understand what forces cause a specific response. That means dinoflagellates could be used as a non-invasive means of understanding fluid flow around swimmers like dolphins or sea lions! (Image and research credit: M. Jalaal et al.; via APS Physics)