Expedition guide and photographer Ryan Newburn captures the ephemeral beauty of the glacial caves he explores in Iceland. These caves are in constant flux, thanks to the run and melt of water. The scalloped walls are a sign of this process of melting and dissolution. The icicles, too, hint at ongoing melting and refreezing. Caves can appear and disappear rapidly; they’re a dangerous environ, but Newburn freezes them in time, letting the rest of us experience a piece of their majesty. See more of his images on his Instagram. (Image credit: R. Newburn; via Colossal)
Tag: ice

“Eternal Spring”
With every spring comes the thaw. Warming temperatures melt winter’s ice, carving it away to reveal the surfaces beneath. Christopher Dormoy’s macroscale timelapse “Eternal Spring” captures this dynamic, showing the process drop-by-drop and rivulet-by-rivulet. It’s also a commentary on melting in general as human-driven climate change chips away at ice that formed over millennia. (Video and image credit: C. Dormoy)

Summer Melt
A warm summer in 2022 has resulted in record melting on Svalbard. Located halfway between the Norwegian mainland and the North Pole, more than half of Svalbard is normally covered in ice. But with glaciers in retreat and firn — a surface layer of compressed porous snow — melting, pale blue ice is getting direct exposure to the sun and warm air temperatures. The result has been melting 3.5 times larger than the average melt between 1981 and 2010. Look closely and you’ll find deep blue meltwater ponds dotting the ice, too. The run-off of meltwater has likely carried extra sediment into the surrounding waters, accounting for some of the paler water colors along the coast. (Image credit: J. Stevens/USGS; via NASA Earth Observatory)

Hydrophobic Ice
Water is an endlessly peculiar substance, eager to adopt many configurations. Each molecule can form up to four, highly-directional bonds. In this study, researchers found an unexpected configuration, a 2D type of ice known as bilayer hexagonal ice, on a corrugated gold surface. Bilayer hexagonal ice has been known since the late 1990s, but it was thought to be comparatively rare. In this form, water molecules assemble in an ice only two molecular layers thick, with hydrogen bonds between neighboring molecules taking up nearly all possible binding sites. With nowhere to bind, additional water cannot add to the ice’s thickness, making the ice as a whole hydrophobic or “water-fearing”.

This illustration shows a type of 2D ice, known as bilayer hexagonal ice, as it forms on a corrugated gold surface. From above (top half), the water molecules align to the surface with some molecules (red) in the troughs and others (pink) along the ridges. Viewed from the side (lower half), most of the molecules bind with their neighbors, leaving few H-bond sites available where more water layers of water could attach. This inability to add more vertical layers is why the ice appears hydrophobic. Previously, this type of ice had only been found on hydrophobic, flat surfaces. In the latest research, though, researchers found that surface corrugations allowed the ice to form, even on a surface that was only slightly hydrophobic. Observations like these help theorists modeling water and its interactions with surface. (Image credit: top – E. McKenna, illustration – APS/A. Stonebraker; research credit: P. Yang et. al.; via APS Physics; submitted by Kam-Yung Soh)

Bendable Ice
Ice — as we typically encounter it — is extremely brittle and easily broken. That’s due to defects in the ice, places where atoms have settled into a spot that does not match the perfect crystalline alignment. Because tiny defect-free threads of ice made by researchers turn out to be wildly flexible!
To make these perfect ice strands, each of which is a tiny fraction of the thickness of a human hair, researchers applied an electric voltage to a needle in a water-vapor-filled chamber. The technique condensed ice microfibers with perfect crystal structures in a matter of seconds. When bent, the microfibers actually shift from one crystalline arrangement to another in order to carry stress, and once the force is removed, the thread reverts back to its initial straight form. (Image and research credit: P. Xu et al.; via Science News; submitted by Kam-Yung Soh)

Columbia Glacier’s Retreat
In southeastern Alaska, the Columbia Glacier once stretched as far as Heather Island in Prince William Sound. After a long period of stability, the glacier began retreating in 1980 and currently sits more than 15 miles from its previous extent. This video explores the glacier’s evolution through false-color satellite imagery, which allows researchers to distinguish the glacier from sea ice, open water, exposed rocks, and nearby vegetation. Though rapid overall, the glacier’s retreat takes place in fits and starts, due to a combination of influences including climate change, sea and ice interactions, and the effects of local topography. (Video and image credit: NASA Earth Observatory)

False-color animation showing the retreat of Alaska’s Columbia Glacier since 1980. 
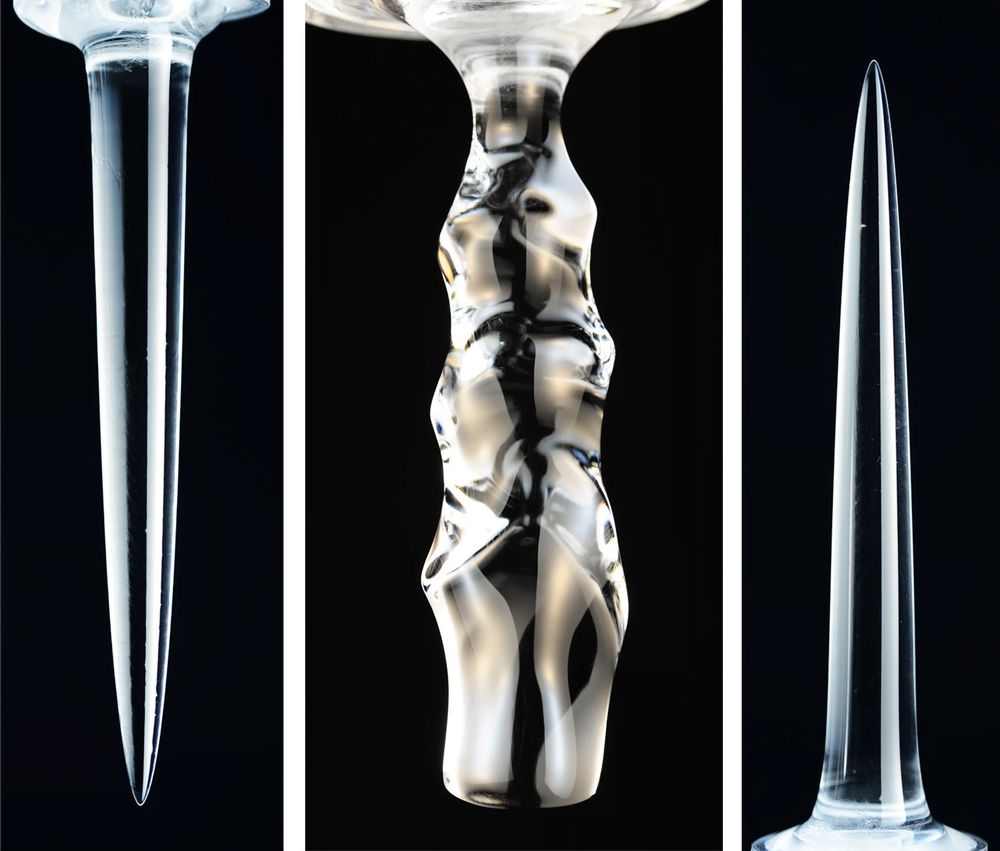
The Shapes of Melting Ice
Water is an odd substance because it is densest at 4 degrees Celsius, well above its melting point at 0 degrees Celsius. This density anomaly means that melting ice takes on very different shapes, depending on the temperature of the water surrounding it. At low temperatures (under 4 degrees Celsius), the cold water melting off the ice is denser than the surroundings, so it sinks. The sinking fluid melts lower portions of the ice faster, leading to an inverted pinnacle (Image 1).
In contrast, at higher temperatures (above 7 degrees Celsius), the meltwater is lighter than the surroundings and therefore rises, creating an upward-pointing pinnacle (Image 3). At intermediate temperatures, some areas of the ice see rising meltwater and some see sinking. This complicated flow pattern sets up vortices that result in a scalloped edge along the ice (Image 2). (Image and research credit: S. Weady et al.; via APS Physics)

Beijing 2022: Ice’s Slideability
As scientists continue to unravel the peculiarities of ice, they’ve found that ice’s friction depends both on the object sliding on it and the ice’s hardness. At extremely low temperatures, water molecules at the ice’s surface are held rigidly by the hard ice, resulting in high friction. At intermediate temperatures, however, water molecules at the surface were more mobile — especially with a quick-moving slider going by — so the friction decreased.
But as the ice approached its melting point, the friction behavior shifted again. As the ice softened, sliding objects could begin to plough into the ice, dramatically increasing contact and friction. When ploughing begins depends on temperature, slider shape, contact pressure, slider speed, and ice hardness.
Beyond the lab, researchers found that weather plays a role in slideability, too, since humidity and air temperature can affect the thickness of the liquid-layer at the ice’s surface. (Image credit: SHVETS Productions; research credit: R. Liefferink et al.; via APS Physics; submitted by Kam-Yung Soh)

Beijing 2022: Monobob
Bobsleigh, as a discipline, has been dominated in recent years by teams seeking every aerodynamic advantage to shave hundredths of a second off their runs. So it’s fascinating that the newest event in the discipline — the women-only monobob — cuts away that secretive part of the sport by permitting sleds from only one manufacturer. Every athlete competes in an identical sled. Not only that, they swap sleds between runs based on their times! So the fastest athlete from the first run will switch sleds with whomever had the slowest time.
The event’s rules refocus the competition on athletic performance and skill rather than incentivizing countries who can afford to spend more money on wind tunnel testing and F1 design companies. That’s a great step toward leveling the playing field. I can’t wait to watch! (Image credit: OIS)

Beijing 2022: Why Are Ice and Snow Slippery?
Although every Olympic winter sport relies on the slippery nature of snow and ice, exactly why those substances are so slippery has been an enduring mystery. Michael Faraday hypothesized in the nineteenth century that ice may have a thin, liquid-like layer at its surface, something that modern studies have repeatedly found.
One recent study used an entirely new instrument to probe the characteristics of this lubrication layer and found that it is only a few hundred nanometers thick. But the fluid in this layer is nothing like the water we’re used to. Instead it has a viscosity more akin to oil and its response to deformation is shear-thinning and viscoelastic, more like the complex fluids in our kitchens and bodies than pure, simple water. They found that using a hydrophobic probe modified the interfacial viscosity even further, which finally provides a hint at the mechanism behind waxing skis and skates.
Fortunately for us, we’ve found plenty of ways to employ and enjoy water’s slipperiness, even as the mystery of it slowly gives way to understanding. (Image credit: M. Fournier; research credit: L. Canale et al.; via Physics World; submitted by Kam-Yung Soh)