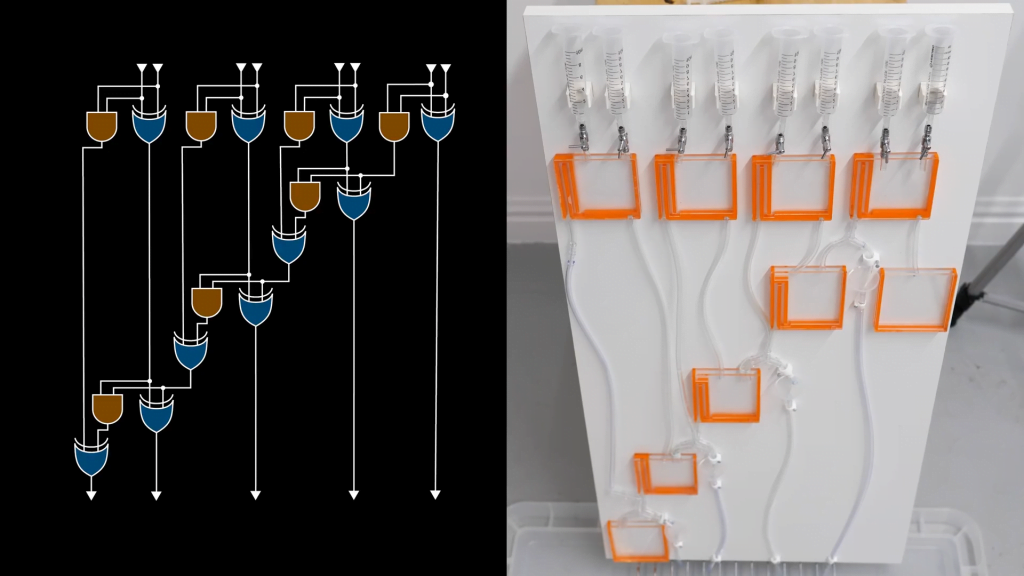
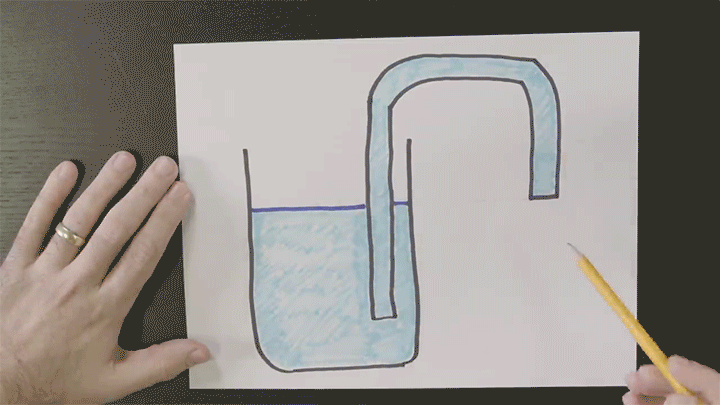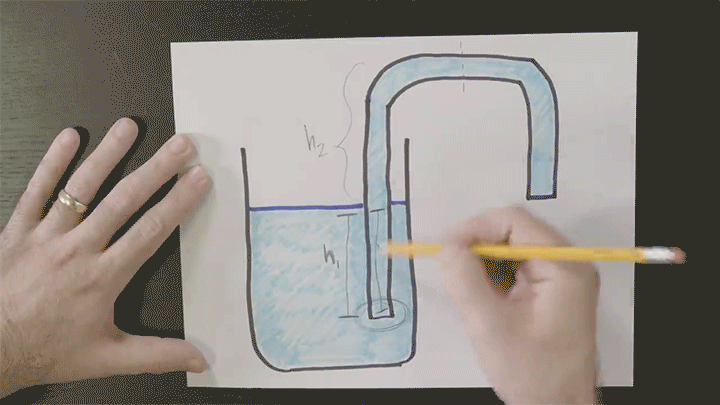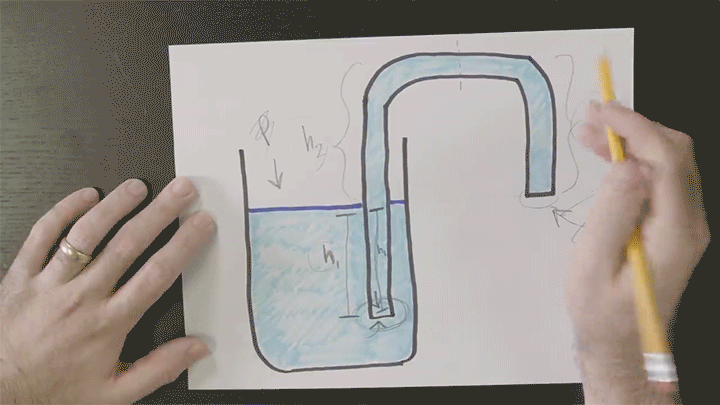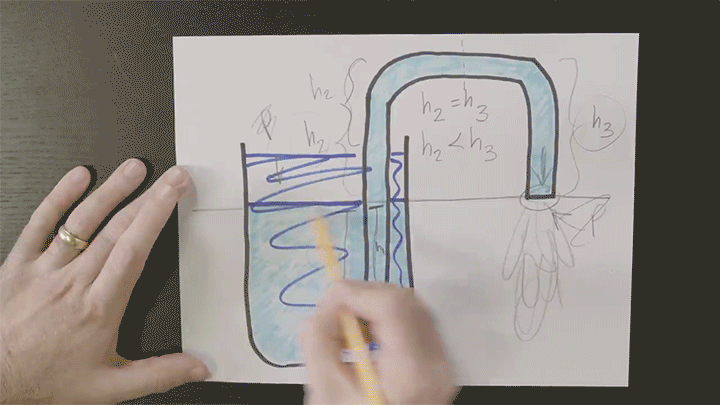Many cultures around the world marinate hard-boiled eggs — like pickled eggs in Europe or tea- and soy-infused eggs from Asia. These delicacies offer a fun (and tasty) way to demonstrate the concept of diffusion, the tendency of a substance to move from areas of high concentration to low concentration via random molecular motion.
Simply steep peeled, hard-boiled eggs in your sauce (or food dye) of choice. Remove an egg every so often and slice it in half to see how far the sauce traveled. You can also play with the temperature to accelerate the diffusion. The longer an egg steeps and the hotter its surroundings, the further into the egg white the sauce will diffuse! (Image credit: Wordridden; research credit: C. Emeigh et al.)