Ngangla Ringco sits atop the Tibetan Plateau, breaking up the barren landscape with eye-catching teal and blue. This saline lake sits at an altitude of 4,700 meters, fed by rainfall, Himalayan runoff, and melting glaciers and permafrost. The lake, like many inland bodies of salt water, has no outflow. Instead, water evaporates from the lake, leaving behind any salts that were dissolved in it. Over time, those left-behind salts build up and make the lake ever saltier. (Image credit: NASA; via NASA Earth Observatory)
Tag: dissolution

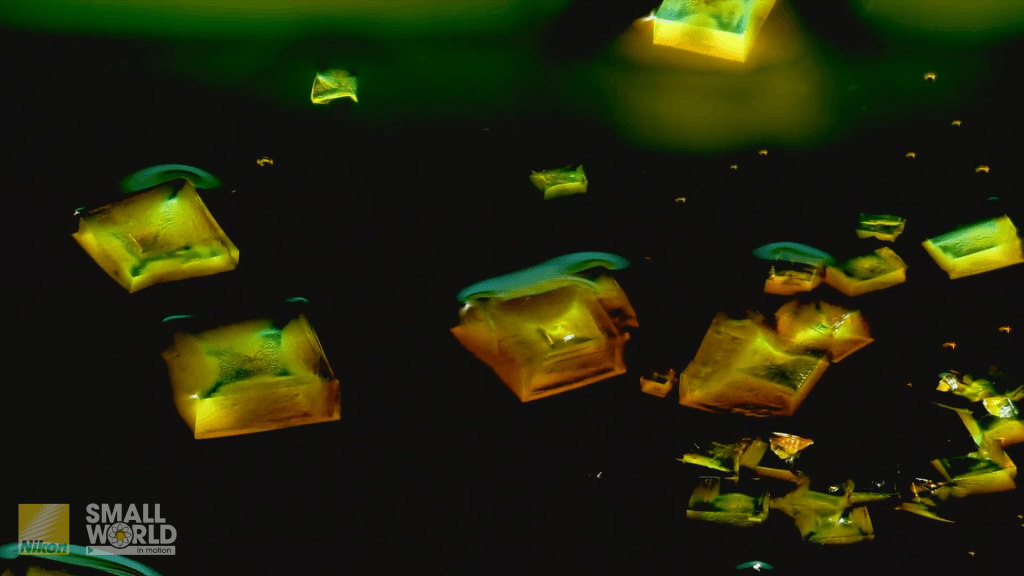
Dissolution and Crystallization
A colorful assortment of salts dissolve and recrystallize in this microscopic timelapse video by retired engineer Jay McClellan. Every step is a gorgeous rainbow of color as the cobalt, copper, and sodium chlorides dissolve, mix, and change. Though we don’t see what’s going on in the water, fluid dynamics are a critical component of both dissolution and crystallization. In the former, concentration gradients change the water’s density, driving buoyant flows. For the latter, crystallization comes out of evaporation, where surface tension often determines where solid particles get left behind. (Video and image credit: J. McClellan; via Colossal)

How CO2 Gets Into the Ocean
Our oceans absorb large amounts of atmospheric carbon dioxide. Liquid water is quite good at dissolving carbon dioxide gas, which is why we have seltzer, beer, sodas, and other carbonated drinks. The larger the surface area between the atmosphere and the ocean, the more quickly carbon dioxide gets dissolved. So breaking waves — which trap lots of bubbles — are a major factor in this carbon exchange.
This video shows off numerical simulations exploring how breaking waves and bubbly turbulence affect carbon getting into the ocean. The visualizations are gorgeous, and you can follow the problem from the large-scale (breaking waves) all the way down to the smallest scales (bubbles coalescing). (Video and image credit: S. Pirozzoli et al.)

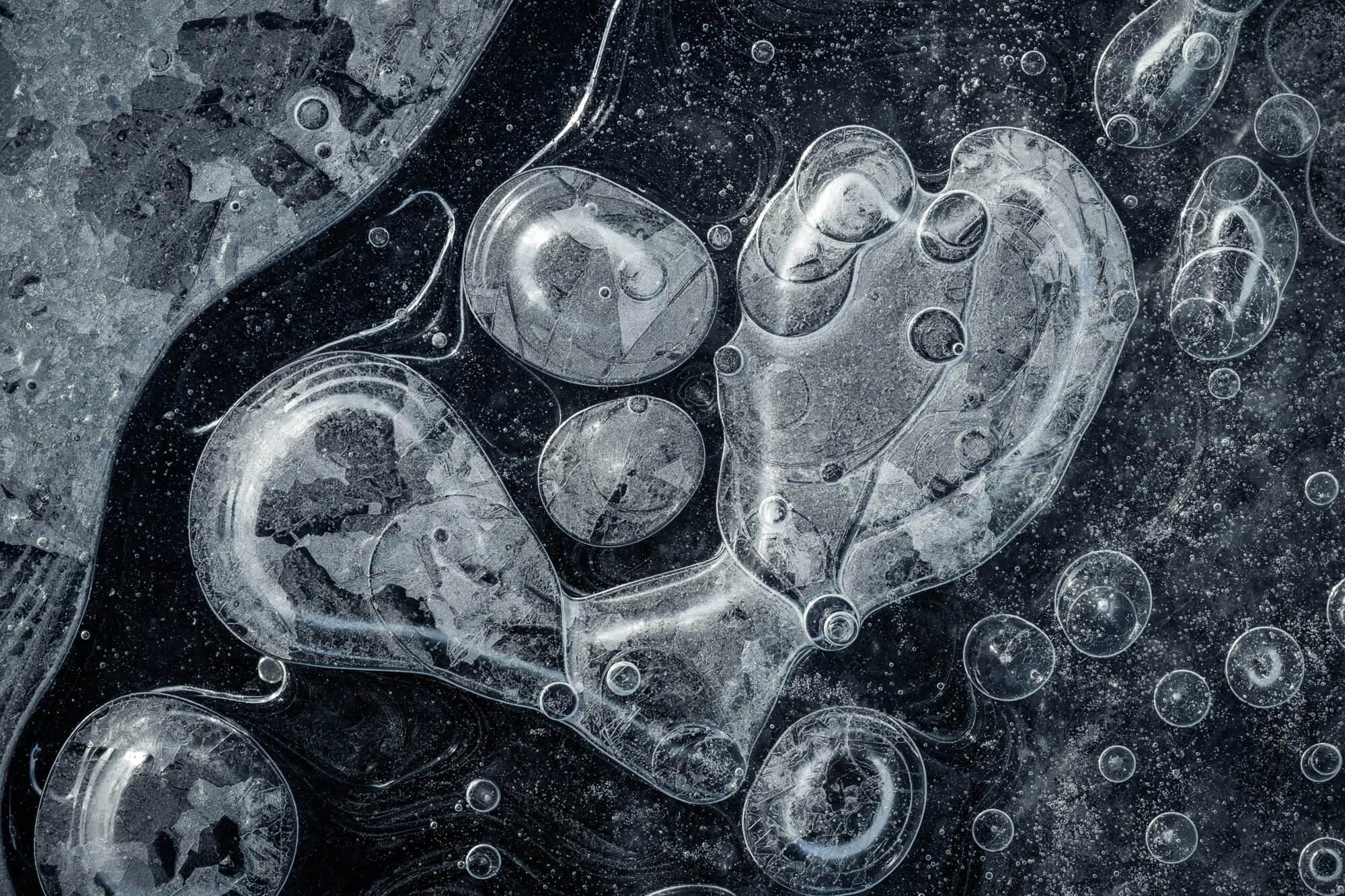
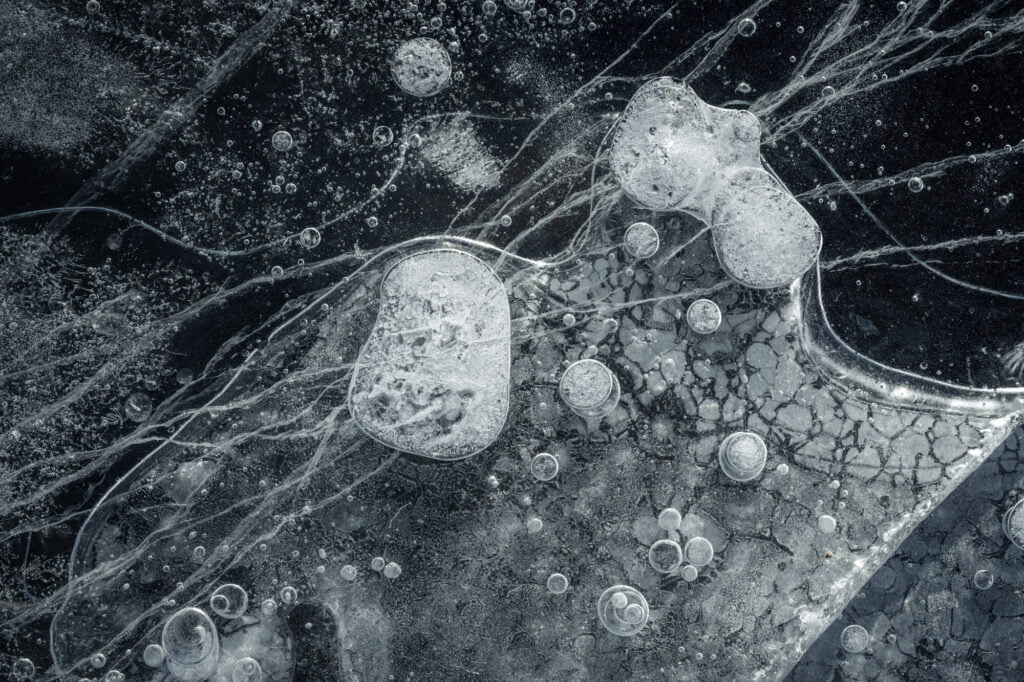
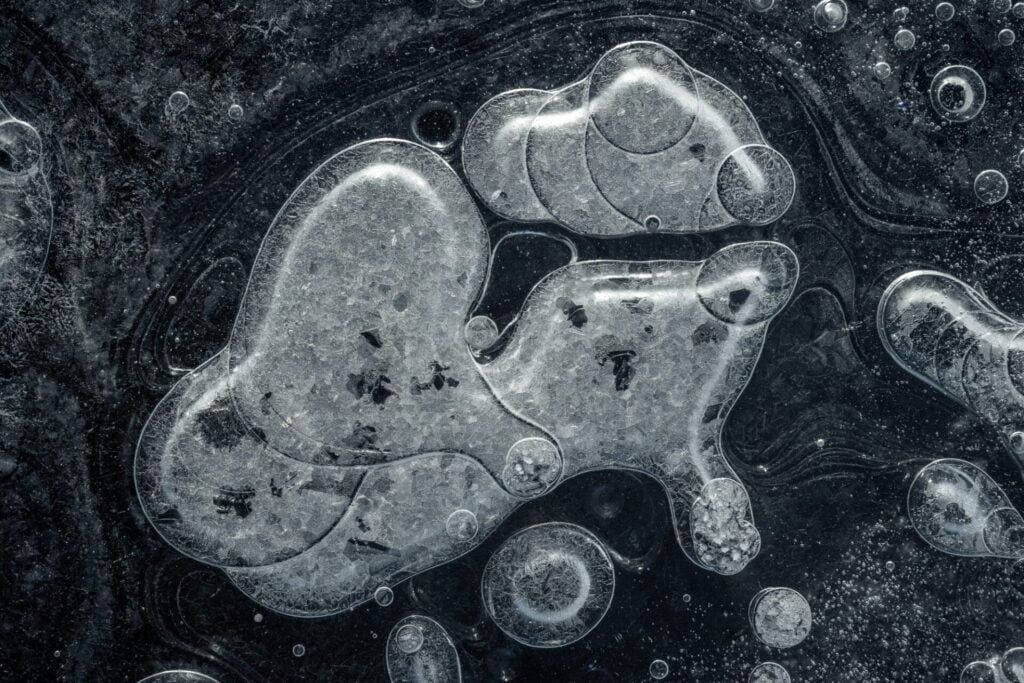
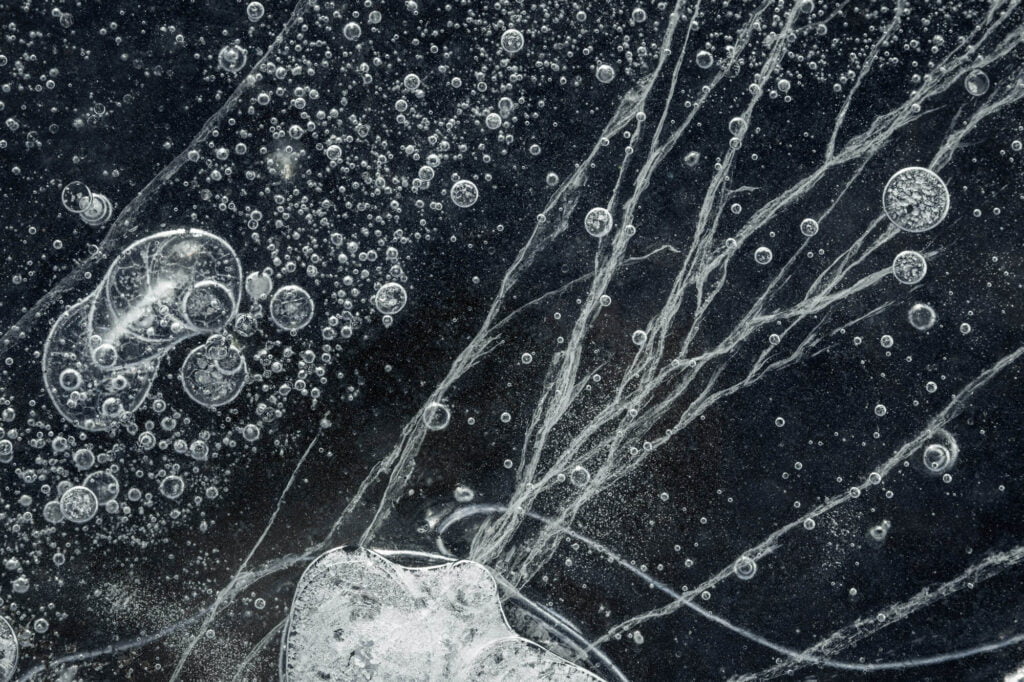
Trapped in Ice
On lake bottoms, decaying matter produces methane and other gases that get caught as bubbles when the water freezes. In liquid form, water is excellent at dissolving gases, but they come out of solution when the molecules freeze. In the arctic, these bubbles form wild, layered patterns like these captured by photographer Jan Erik Waider in a lake on the edge of Iceland’s Skaftafellsjökull glacier. Unlike the bubbles that form in our fridges’ icemakers, these bubbles are large enough that they take on complicated shapes. I especially love the ones that leave a visible trail of where the bubble shifted during the freezing process. (Image credit: J. Waider; via Colossal)

Melting Permafrost Stains Alaskan Rivers Orange
The swiftly melting permafrost of the Arctic is releasing toxic metals like zinc, cadmium, and iron into Alaskan waterways. The contaminant levels are so high that it’s staining many rivers orange — a feature that can be seen from space. A new study identified at least 75 affected rivers in the Brooks mountain range.
In addition to staining the rivers, these metals make the water acidic, with some waterways reaching a pH as low as 2.3, similar to the acidity of vinegar. The combination is deadly to aquatic life in the rivers, and the acidity, unfortunately, will accelerate the dissolution of rocks that can release even more metals into the water. (Image credit: K. Hill/National Park Service; research credit: J. O’Donnell et al.; via LiveScience; submitted by Emily R.)

A contaminated portion of the Kutuk River runs orange alongside an uncontaminated portion of the same waterway. 
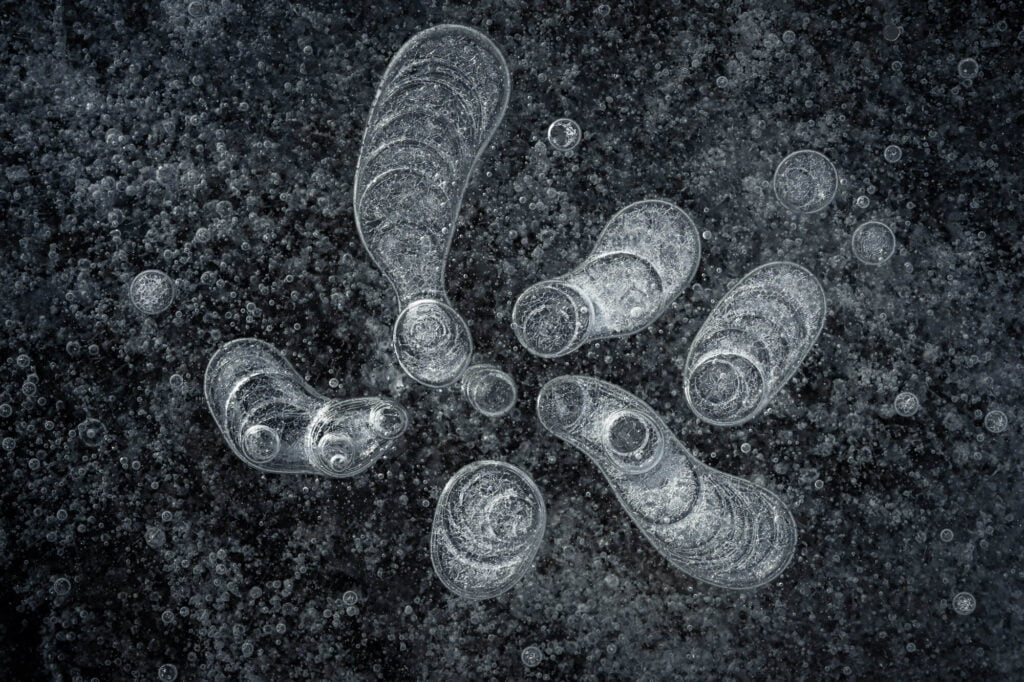
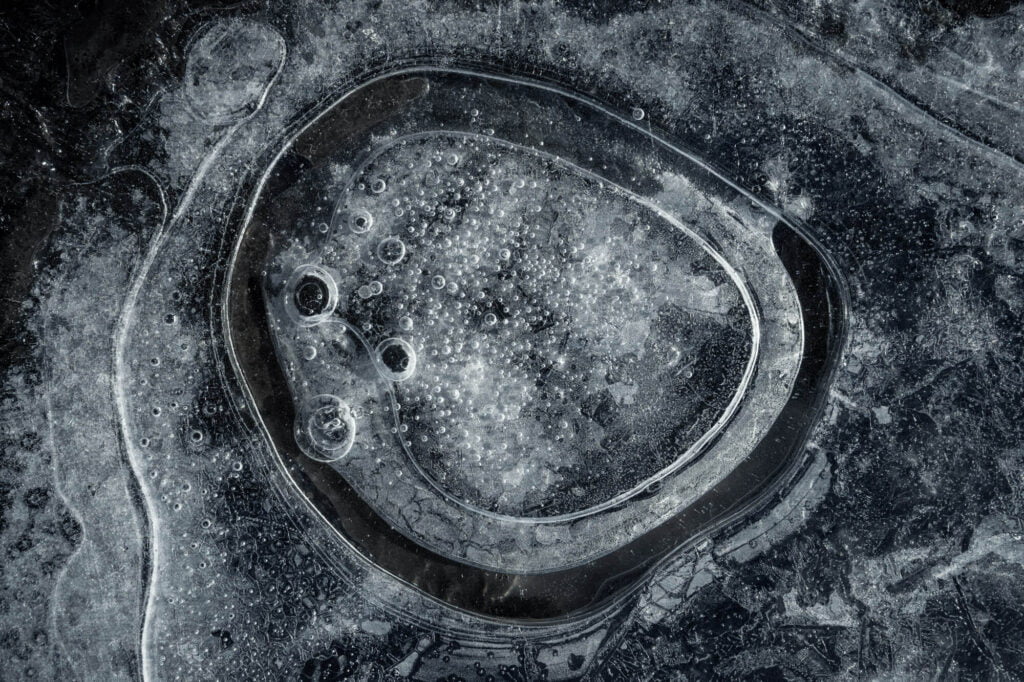
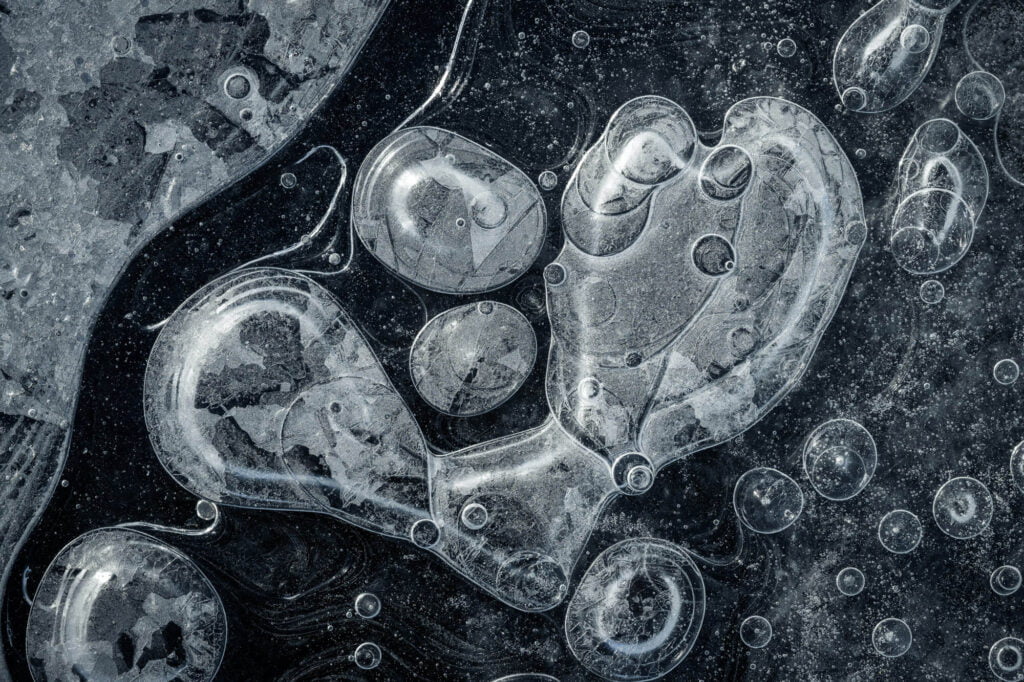
Bubbles Encased in Ice
If you’ve ever made ice in a freezer, you’ve probably noticed the streaks of frozen bubbles inside the ice. In its liquid state, water is good at dissolving various gases — like the carbon dioxide in sparkling water. During freezing, though, those gases cannot remain in solution; the water simply doesn’t have space between its crystalline ice lattice for non-water molecules. So the gases are forced out of solution, where they form bubbles. The final shape of the frozen bubble depends on the interplay between the speed of a bubble’s growth and how quickly the ice freezes. Here, the researchers used polarized light to outline the bubbles in color, highlighting the wide array of possible shapes. (Image credit: J. Meijer and D. Lohse; via GoSM)

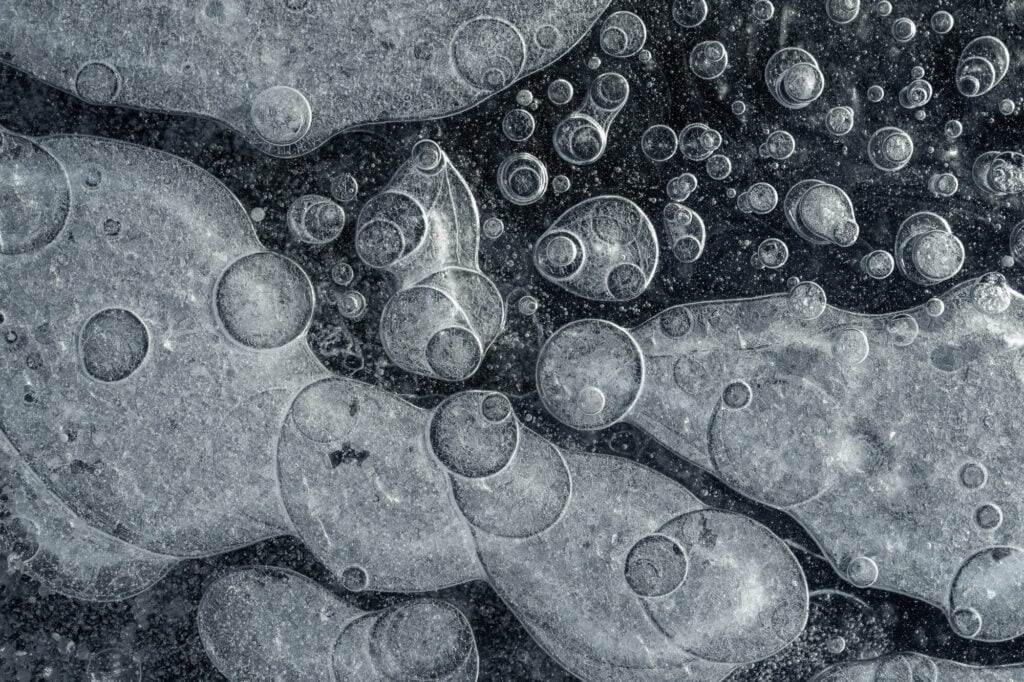
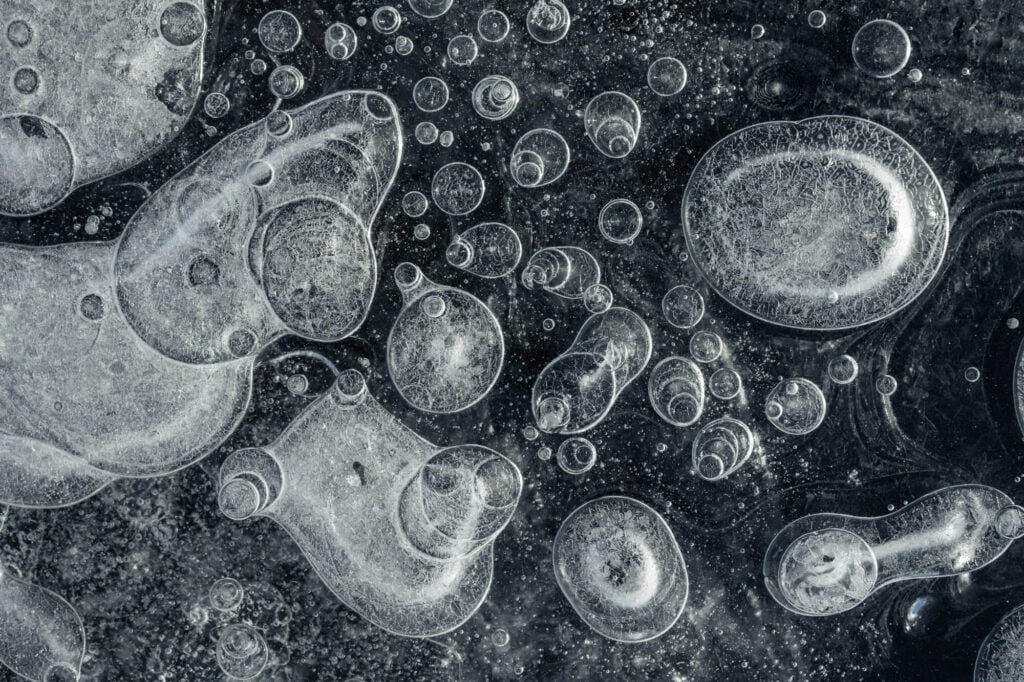
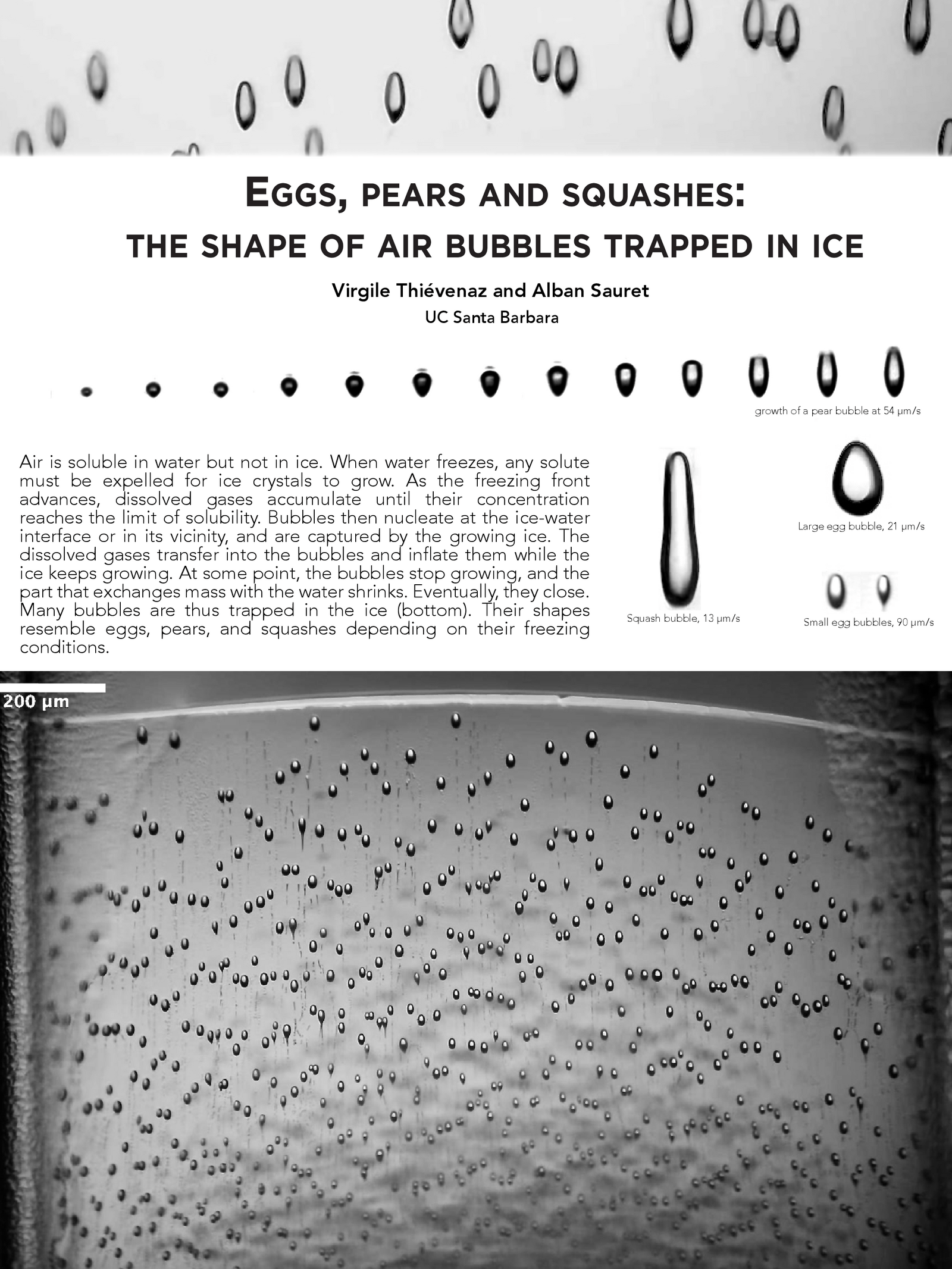
Frozen in Ice
Air can dissolve in water, but not in ice. So as water freezes, any dissolved gases have to get squeezed out in order for the ice crystals to grow. Once the concentration of gases is high enough, a bubble nucleates and gets captured by the growing ice around it. The shape of the final bubble depends on its freezing conditions. As seen here, bubbles take on all kinds of shapes, ranging from egg-like to a long and skinny squash-like shape. (Image credit: V. Thiévenaz and A. Sauret)

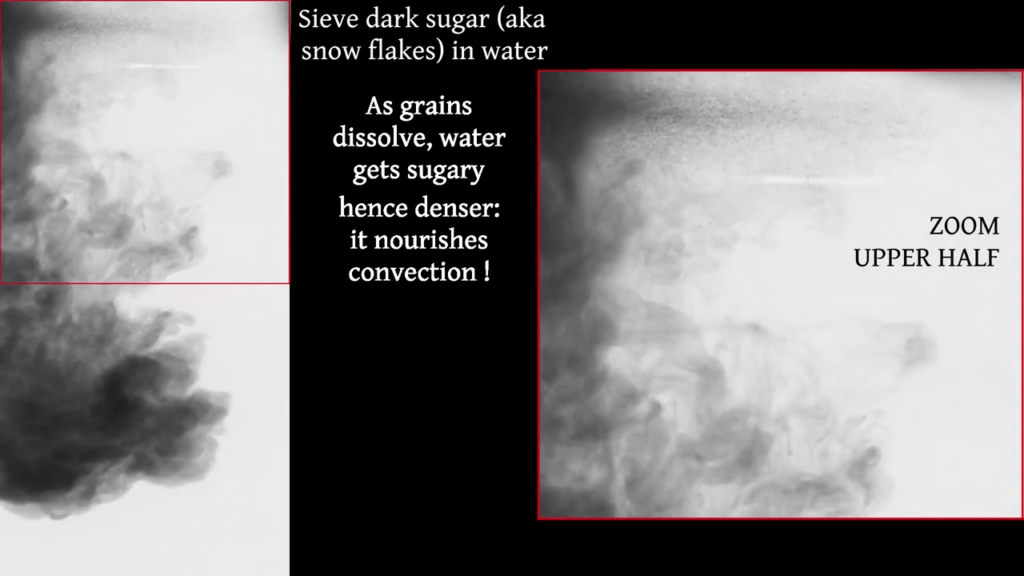
Snowing in the Core
Some rocky planetary bodies, like Jupiter‘s moon Ganymede, generate magnetic fields through snow-like, solid precipitation that falls in their liquid metal cores. To study this peculiar and complex arrangement, researchers look at sugar grains falling through — and dissolving into — water. The solid sugar grains mimic the iron snowflakes that fall in Ganymede’s core. As they sink, they drag fluid with them. But the grains can also dissolve, making the fluid around them denser and prone to sinking even faster. The dense, sinking flows trigger buoyant convection inside the surrounding fluid.
As seen in the experiments, there are many factors competing here. Large grains dissolve more slowly and are able to drag more fluid with them as they fall. Small grains, on the other hand, dissolve quickly, causing more buoyancy-driven flows. Laboratory analogs like these help scientists unravel the complexities of situations we cannot observe otherwise. (Image and video credit: Q. Kriaa et al.)

Vietnam’s Emerald Isles
Vietnam’s Hạ Long Bay is home to more than 1,600 islands, many of them made up of mountainous limestone. The area is famous for its karst features, a type of terrain formed from highly porous, water-soluble rock. Over time, water dissolves and fractures the limestone, creating karst landscapes full of caves, springs, sinkholes, and fluted rock outcroppings. The area’s erosion also produces highly fertile soil, leading to a verdant ecosystem with many unique and endemic species. (Image credit: N. Kuring/NASA/USGS; via NASA Earth Observatory)

Watery Salt Flats
Unusually high rainfall in Bolivia’s Salar de Uyuni turned the world’s largest salt flat into a shallow salt lake. These natural-color satellite images show the area in late January 2022. If you zoom in on the full resolution image, there are incredible detailed swirls in the water. It’s like peering at an abstract or Impressionist painting. The many colors are attributable to several sources, including volcanic sediments, runoff, and a variety of microbes and algae thriving in the mineral-filled waters. (Image credit: L. Dauphin; via NASA Earth Observatory)