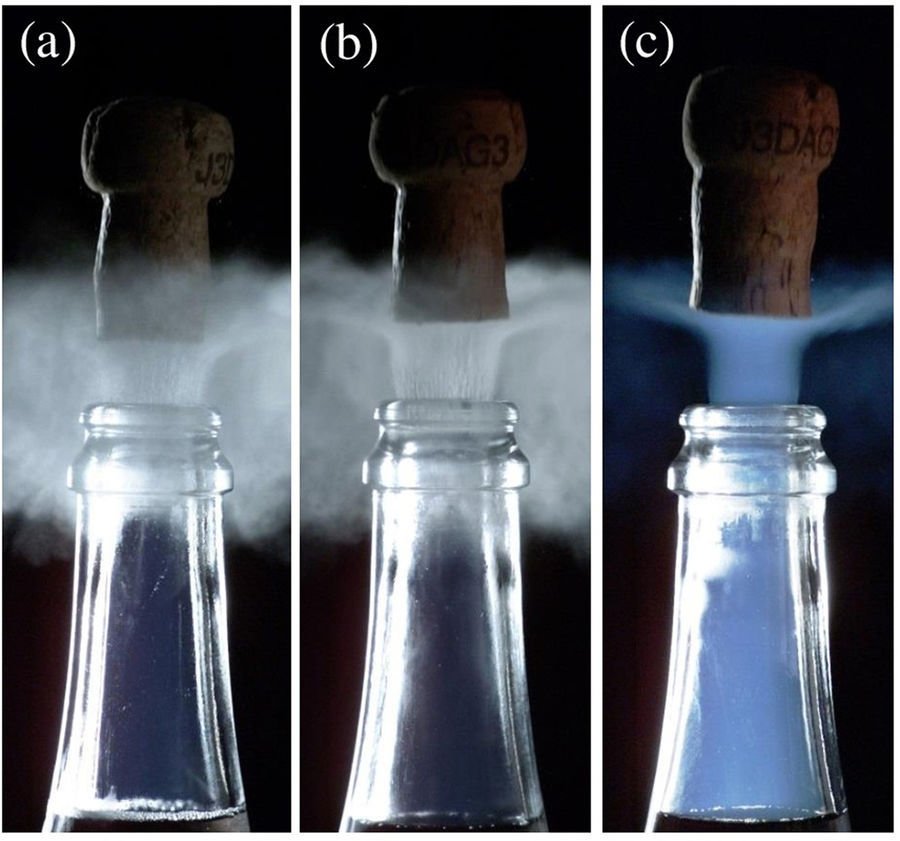
Watch the bubbles rising in a glass of champagne and you’ll see them form tiny straight lines, with each bubble following its predecessor. But in a carbonated soda, the bubbles rise all over the place, each following its own zig-zaggy line. Why the difference? A recent study points out the culprits: bubble size and surfactants.

Looking at a variety of beverage scenarios, researchers found that both a bubble’s size and its surfactant concentration affected what sort of path it followed. For clean (surfactant-free) bubbles, small bubbles take a winding path, but bigger ones move in a straight line. Simulations show that bubbles can only form a straight path if they produce enough vorticity on their surface. Small bubbles just can’t deform enough to do that.

When surfactants get added, though, the story changes. For bubbles of a set size, adding surfactants made their paths straighter. This was due, the team found, to a bump in vorticity provided by the stabilizing effect of the surfactants. Champagne, they concluded, has straight bubble paths despite its tiny bubbles because of the drink’s high number of flavorful surfactants. (Image credit: top – D. Cook, experiments – O. Atasi et al.; research credit: O. Atasi et al.; via APS Physics)