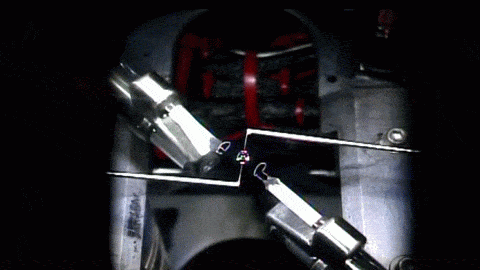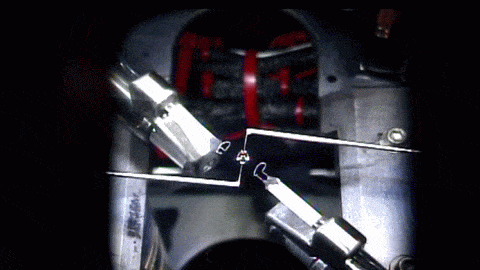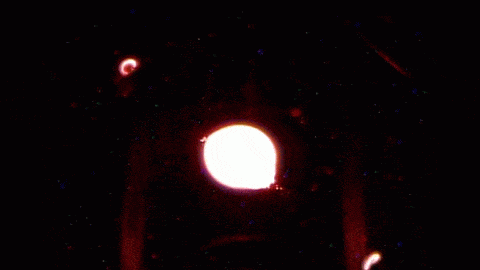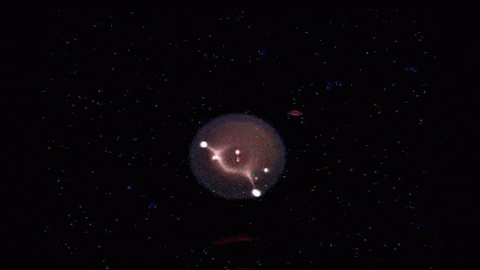
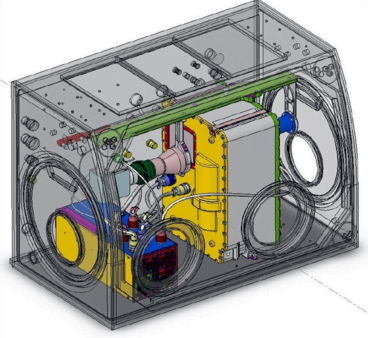
In the playground of microgravity, every day processes can behave much differently. This photo comes from the RUBI experiment, the Reference mUltiscale Boiling Investigation, aboard the International Space Station. Freshly installed and switched on, the apparatus is now generating bubbles like this one. On the left, you see temperature sensors used to measure bubble temperatures. High-speed and infrared cameras are also part of the experiment.
The advantage of studying boiling in space is a lack of gravity that can mask or overwhelm subtler effects. It effectively slows down the process, making it easier to observe. And since boiling is such an important part of heat transfer in many manmade devices, it shows us how we have to adapt when operating in an environment where heat – and bubbles – don’t automatically rise. (Image credit: ESA; submitted by Kam-Yung Soh)