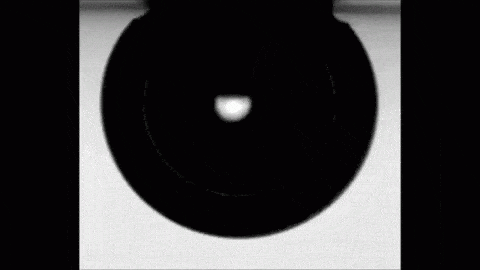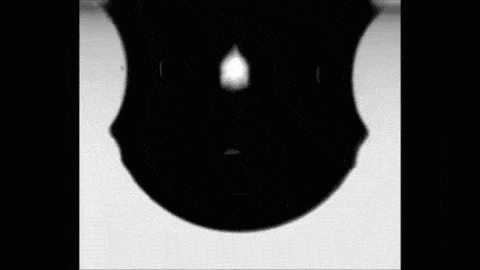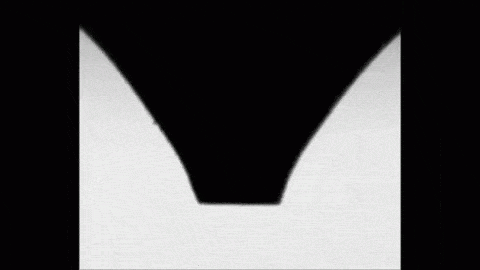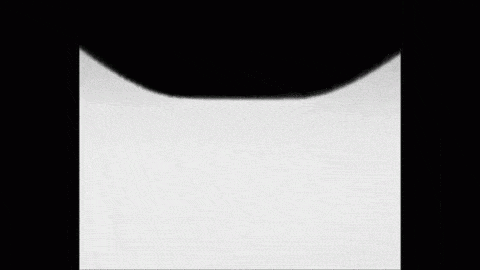
Place a drop of carbonated water on a superhydrophobic surface and it will slide almost frictionlessly, much the way Leidenfrost drops do. The drop behaves this way thanks to the self-produced layer of carbon dioxide vapor that it levitates on. As the gas escapes, the drop eventually settles back into contact with its surface. But until then, its levitation makes for some fun.

Single droplets aren’t the only source of fun, however. In the images above, researchers coated the left half of a wine glass with a superhydrophobic treatment, while leaving the right half of the glass untouched. Once (dyed) carbonated water is poured into the glass, we see a bizarre dichotomy. In the right, untreated half of the glass, carbon dioxide bubbles nucleate, grow, and rise through the glass. But on the left side, the liquid appears still and bubble-less. In fact, the carbon dioxide gas on the left side is forming a continuous bubble film by the surface of the glass! (Image, video, and research credit: P. Bourrianne et al., see also)