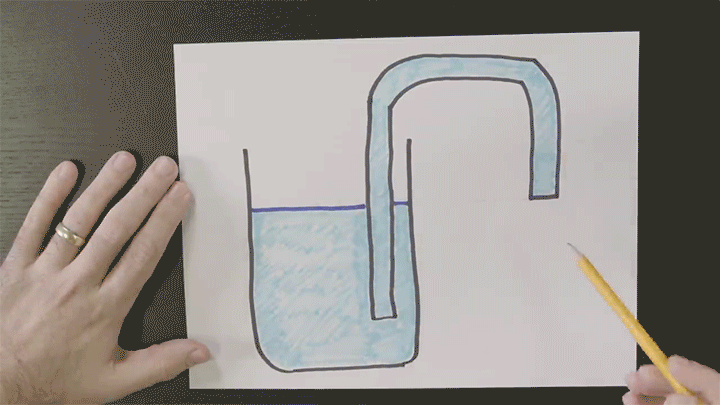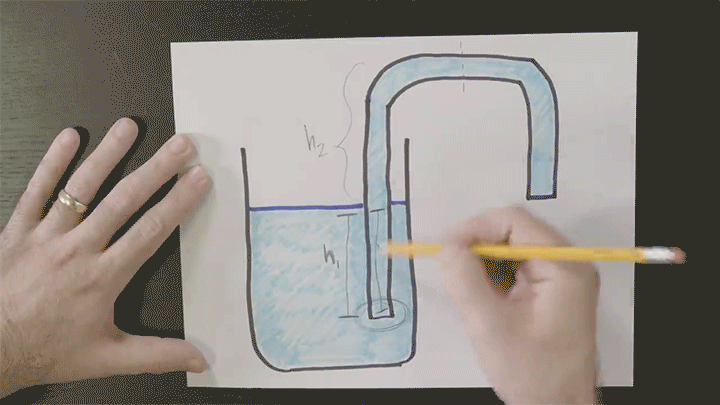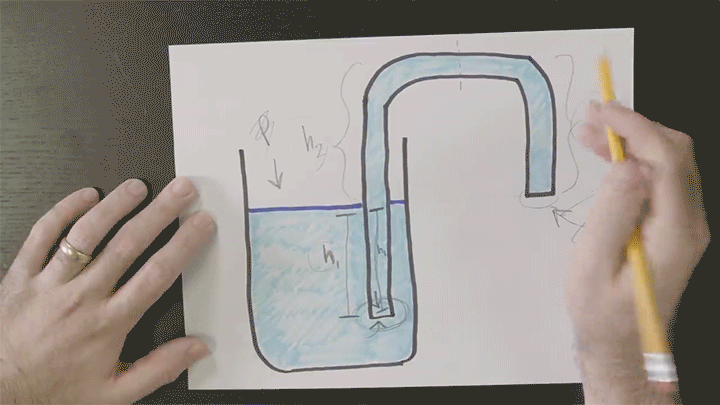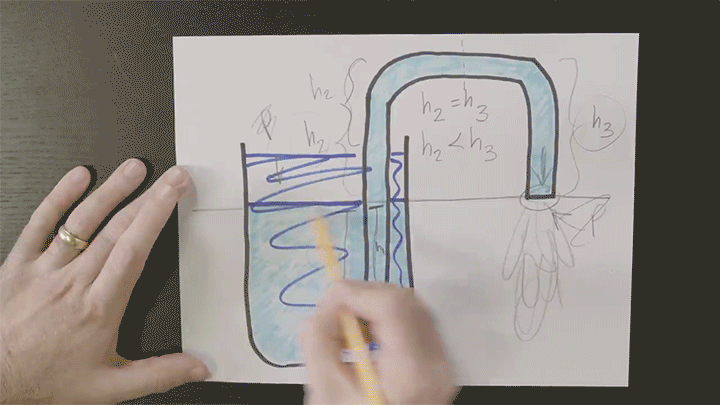Last November, a windstorm, known as Storm Ciarán in the U.K., blew through Europe with wind speeds as high as 130 kilometers per hour. All that wind came with a significant drop in atmospheric pressure. Researchers found that the pressure drop was large enough to lower the boiling point of water more than full 2 degrees Celsius. That difference probably wouldn’t register for anyone waiting for their kettle to boil, but it could decidedly affect the final cup of tea. Tea flavor is quite sensitive to the temperature of the boiling water used to brew it, as it affects how well the tannins get extracted. According to the researchers, Ciarán’s conditions potentially ruined millions of cups of breakfast tea in the greater London area. (Image credit: E. Akyurt; research credit: G. Harrison et al.; via Gizmodo)
Tag: pressure

Playing With Water in 2D Containers
Once again Steve Mould is putting his prototyping skills to use to work out what goes on inside tricky containers. Here he looks at a “magic” wizard’s cup where — like the assassin’s teapot — cleverly placed holes in the side of the cup can block or allow air’s escape. In the wizard’s cup this lets the wizard refill the cup at will.
He also takes a look at how draining works, using tracer particles and a video editing effect that “echoes” previous frames in a video. For the tracer particles, this algorithm effectively visualizes pathlines in the flow. Areas with faster-moving fluid have longer pathlines that are closer together, whereas slow-moving regions have short pathlines. (Video credit: S. Mould)

Making Yeast-Free Pizza
Yeast is a key ingredient in many pizza doughs; as the yeast ferment sugars in the dough, they produce carbon dioxide which bubbles into the dough, creating the light and airy texture necessary for a good crust. It’s a slow process, though, often requiring several hours for the dough to rise. Recently, researchers studied an alternative pizza-making method that generates bubbles in the dough via pressurization — with no yeast required.
The new technique is similar to the process used to carbonate sodas. The team mixed flour, water, and salt and placed the dough in an autoclave, which allowed them to control both temperature and pressure during baking. They dissolved gas into the dough at high pressure and then carefully released the pressure during baking, allowing the bubbles to grow. They used rheological measurements to compare the characteristics of yeasted and yeast-free doughs at various stages in the leavening and baking processes.
Now that they have the methodology down, they’ve purchased a food-grade autoclave and are looking forward to taste testing their yeast-free creations — none more so than their team member who has a yeast allergy! Since the pressures required for their method are quite mild, they hope it’s a technique that restaurants will take on. (Image credit: B. Huff; research credit: P. Avallone et al.)

The Assassin’s Teapot
The assassin’s teapot is a cleverly designed container that can pour from different reservoirs depending on how it’s held. Steve Mould digs into the physics in this video, and he builds a transparent cutaway version of the pot to show exactly how it works. This design uses two separate reservoirs, each with two holes — one in the spout and one concealed near the pot’s handle. By covering this breather hole, the server blocks air from flowing into the teapot, which also keeps the liquid inside from flowing out.
What holds the liquid in? Air pressure, with an assist from surface tension. Atmospheric pressure is enough to hold the fluid inside the pot, provided air has no separate way in. To get in through the spout, air would have to push into the pot at the same time as water coming out. Surface tension prevents that, though, because the spout is too narrow. The same physics keeps water inside a larger bottle with a wire mesh over its mouth. The mesh’s tiny holes are smaller than the capillary length of water, which is the length scale at which surface tension and gravity balance one another. As long as the spout and holes are smaller than that length, surface tension will keep the liquid from deforming enough to get out. (Video and image credit: S. Mould)

Pressure At The Dam
Hydrostatic pressure in a fluid is based on the fluid’s depth. You’ll rarely see a more dramatic example of that power than with a water release from a dam. Here we see the outlet of the Verbund Hydro Power dam in Austria. With 190 meters of water behind the dam, the outlet jet is massive. It moves 20,000 liters of water per second at a speed of 50 meters per second. Imagine what it would be like to stand next to that! (Image and video credit: Discovery UK; submitted by Olwyn B.)

A Primer on Blood Pressure
Some of the most important fluid dynamics goes on every moment inside our bodies. After only a few weeks of gestation, the human heart begins its lifelong task of pumping blood throughout tens of thousands of kilometers’ worth of blood vessels. One of our simplest methods for tracking the health of this critical system is a person’s blood pressure, which measures the forces exerted on our blood vessels as our hearts pump. This video gives a brief primer on blood pressure as well as some of the problems that arise when extended bouts of high blood pressure damage our blood vessels. (Image and video credit: TED-Ed)

Self-Started Siphoning
Here’s a fun activity you can do while you #StayHome: build a self-starting siphon. Michael from VSauce explains how in this video. Moving fluids from one location to another is almost always about pressure, and a siphon is no different. To get the water to flow, there must be unequal pressures driving the liquid to move from high pressure to lower pressure. This is the basic physics behind any siphon; the fun of a self-starting siphon comes from generating enough pressure imbalance to start flow without applying suction. (Video credit: D!NG/M. Stevens)

The Power of a Penguin’s Rectum
When brooding their eggs, penguins can rarely leave the nest, but answering nature’s call is still necessary. To keep the nest clean, Adélie penguins project their feces up to more than a meter away. A new study refines previous calculations on this subject and finds that the penguin’s rectum develops far higher pressures than that of humans.
In one hypothetical calculation, the authors estimate that a human of average height, capable of developing penguin-like rectal pressures, would project excrement more than 3 meters. In the authors’ words, “He/she should not use usual rest rooms.”
Knowing the likely range of contact for penguins is important primarily for zookeepers, who understandably would like to avoid such projectiles. (Image credit: H. Neufeld; research credit: H. Tajima and F. Fujisawa; via phys.org)

Tranquilizer Darts in Slow Mo
Like most syringes, tranquilizer darts use pressure to drive flow. But where a typical syringe has that pressurization provided by a human driving the piston, tranquilizer darts must deploy without any hands-on action. As shown in the video above, this is achieved by pressurization prior to firing.
The tranquilizer dart has a few key features. Its needle, though sharp, does not have a hole in the end. Instead, it has a hole partway down the barrel of the needle, which is covered before launch by a rubber sleeve. The dart also contains two chambers. One is filled with the medicine being deployed. The other gets pressurized with air through a one-way valve. As long as the rubber sleeve stays over the needle’s hole, the dart is then pressurized, but the fluid has nowhere to go.
Until it’s fired, of course. On impact, the rubber sleeve is pushed away, and the higher pressure inside the air chamber drives the medicine out of the needle and into the animal. (Video and image credit: The Slow Mo Guys)

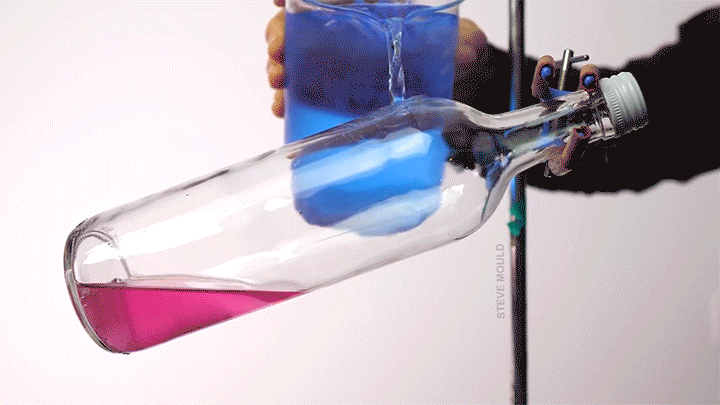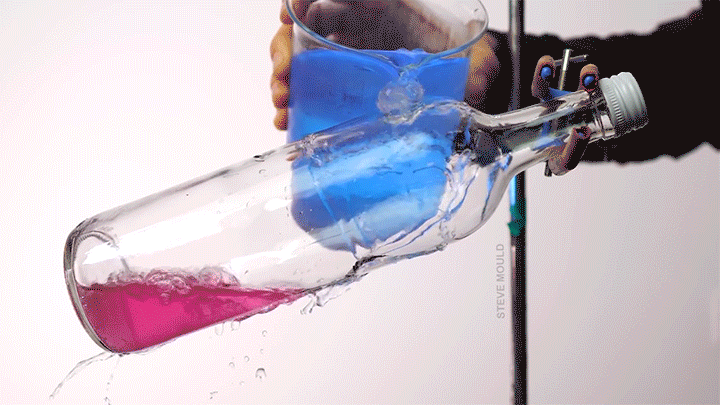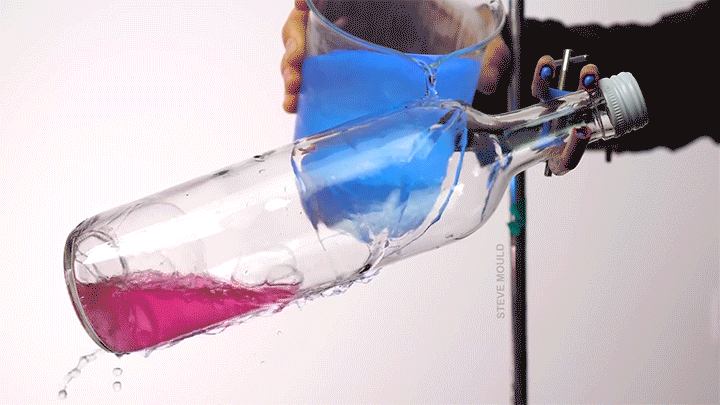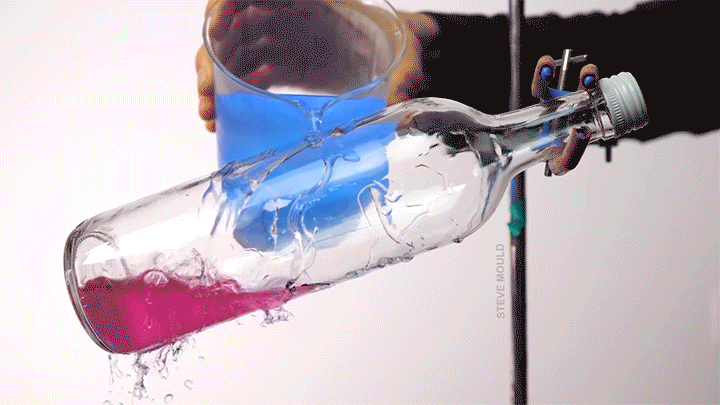
Boiling Water Using Ice Water
Steve Mould demonstrates a neat thermodynamic trick in this video by using ice water to boil hot water. The key to understanding this is recognizing that the boiling point of water depends both on its temperature and its pressure.
Here’s the set-up (which, to be clear, neither he nor I recommend you try yourself): microwave some water in an open bottle until the water is hot enough to boil. Remove the bottle from the microwave and screw on the lid. At this point, you’ve confined any water vapor coming off the hot water, thereby raising the pressure inside the bottle. Even though it’s still quite hot, the water will stop visibly boiling.
Now pour ice water over the top of the bottle. Because water vapor has a lower heat capacity than liquid water, this will preferentially cool the vapor. As its temperature drops, its pressure will also drop. Liquid water boils at lower temperatures when the pressure is lower. (This is part of why cooking and baking instructions are quite different in Denver than they are in Miami.) When the internal pressure in the bottle drops, the remaining hot water will start to visibly boil. (Image and video credit: S. Mould)