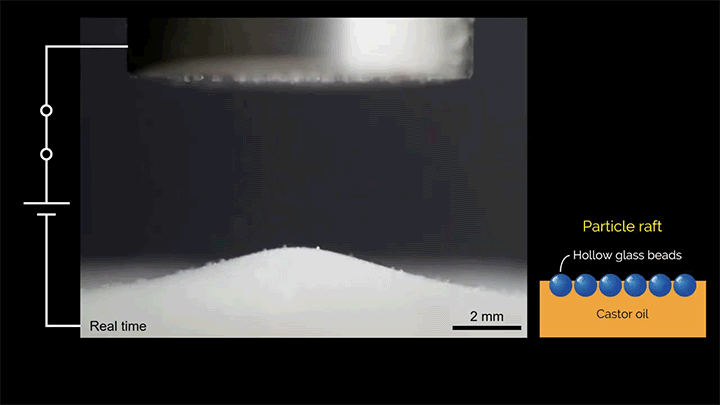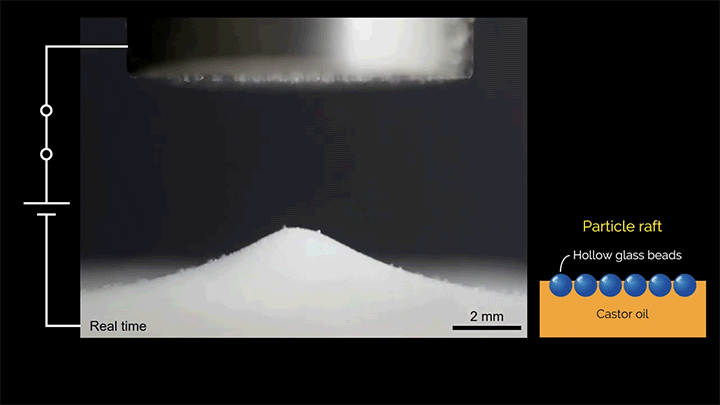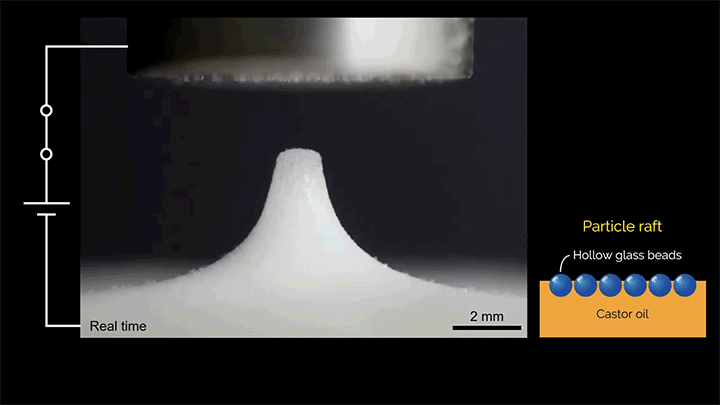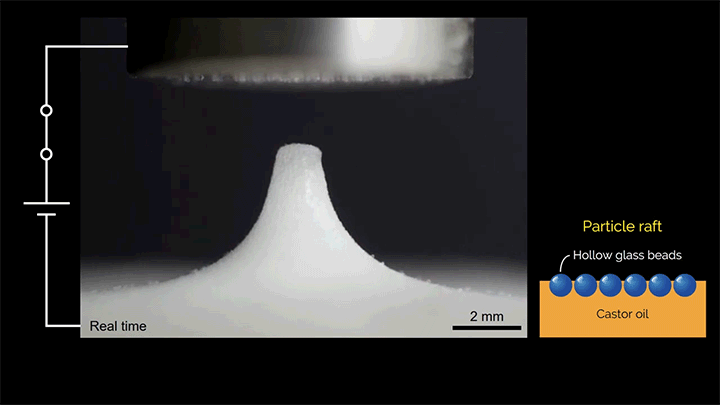
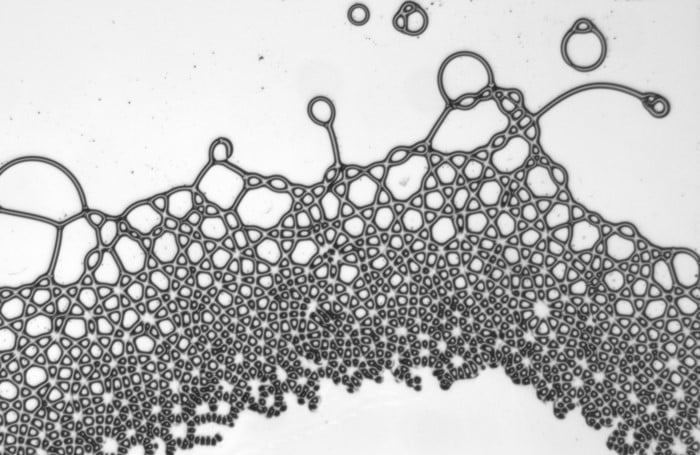
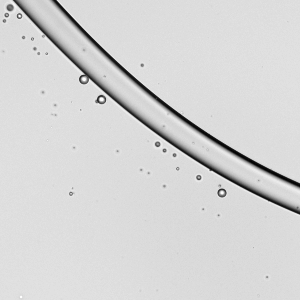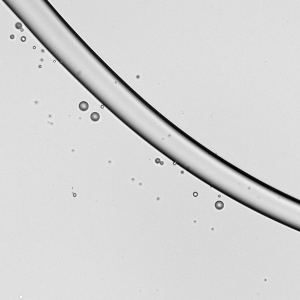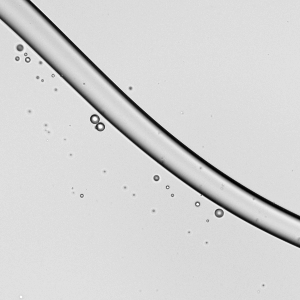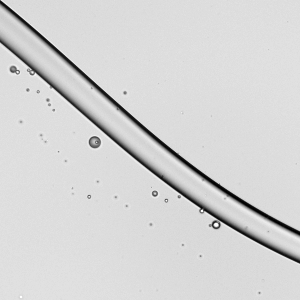
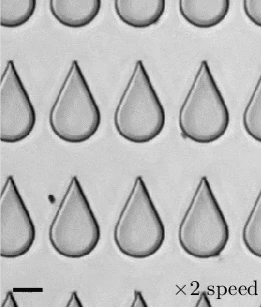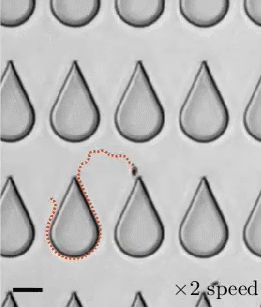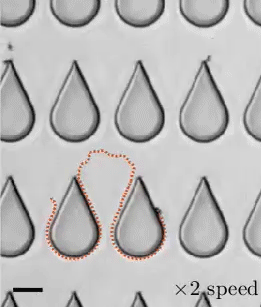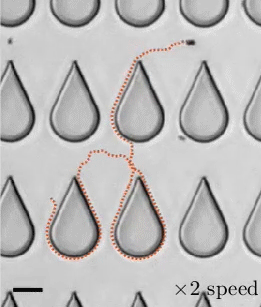
When a droplet falls on a surface, it spreads itself horizontally into a thin lamella. Sometimes — depending on factors like viscosity, impact speed, and air pressure — that drop splashes, breaking up along its edge into myriad smaller droplets. But a new study finds that a small electrical charge is enough to suppress a drop’s splash, as seen below.

The drop’s electrical charge builds up along the drop’s surface, providing an attraction that acts somewhat like surface tension. As a result, charged drops don’t lift off the surface as much and they spread less overall; both factors inhibit splashing.* The effect could increase our control of droplets in ink jet printing, allowing for higher resolution printing. (Image and research credit: F. Yu et al.; via APS News)
*Note that this only works for non-conductive surfaces. If the surface is electrically conductive, the charge simply dissipates, allowing the splash to occur as normal.