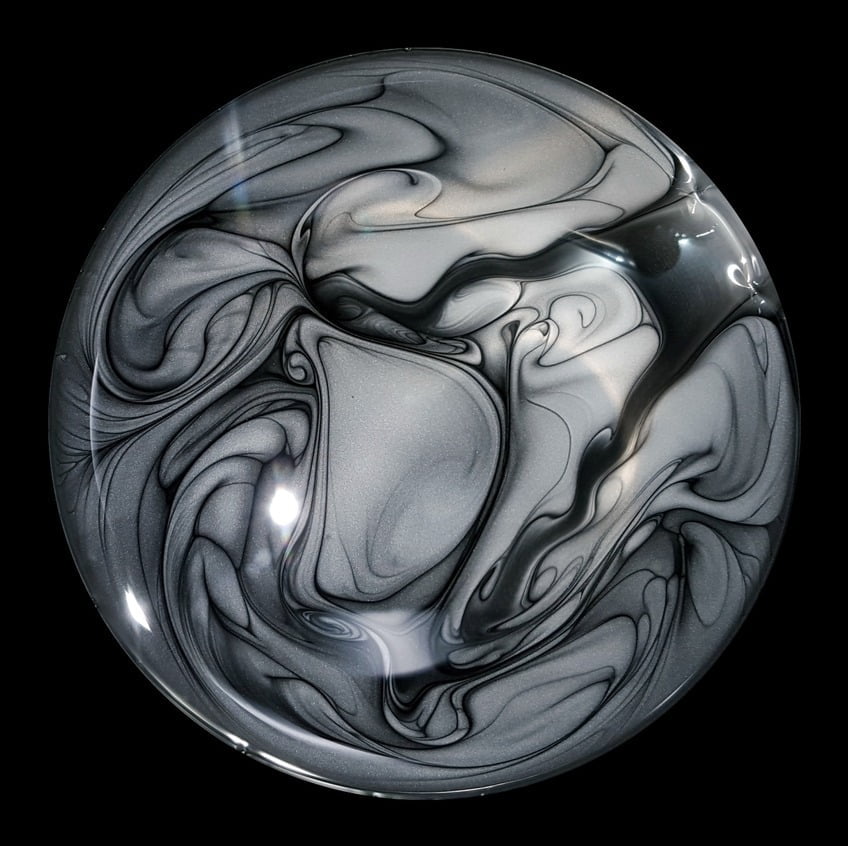
Graphene powder swirls in alcohol in this prize-winning photo from this year’s Engineering and Physical Sciences Research Council photography competition in the UK. The image was captured while producing graphene ink that can print circuits directly onto paper. According to the researcher’s description, this ink is forced through micrometer-sized capillaries at high pressure to rip the layers apart and produce a smooth, conductive ink in solution. In this photo, we seem to see more conventional mixing driven by the powder’s injection and the variations in surface tension due to the alcohol and its evaporation. The graphene leaves behind beautiful streaklines that highlight its path as it mixes. (Image credit: J. Macleod; via Discover)
Tag: marangoni effect

Stabilizing Films
Liquids don’t typically survive very long as thin films. If you try to make one from water, gravity drains it away immediately. (Not so in space.) To make a liquid film stick around, we add surfactants like soap. These extra molecules congregate at the surface of the film and provide a stabilizing force to oppose gravity’s drainage. Exactly what that stabilizing force is depends on the surfactant.
Surfactants that are insoluble are often quite viscous. These molecules distribute themselves across the interface and then they stay. They resist both gravity or even just moving thanks to their high viscosity. That produces a soap film pattern like the one on the right – symmetric and slow to change.
Other surfactants may be soluble in the film and have no appreciable viscosity themselves. These surfactants constantly move and shift on the interface as surface tension variations occur. When weak spots form, the surfactant molecules shift, via the Marangoni effect, to stabilize the film. This creates a film pattern like the familiar one on the right, with an ever-shifting palette of colors. (Image and research credit: S. Bhamla et al., source; submission by S. Bhamla)

The Coalescence Cascade and Surfactants
Drops of a liquid can often join a pool gradually through a process known as the coalescence cascade (top left). In this process, a drop sits atop a pool, separated by a thin air layer. Once that air drains out, contact is made and part of the drop coalesces. Then a smaller daughter droplet rebounds and the process repeats.
A recent study describes a related phenomenon (top right) in which the coalescence cascade is drastically sped up through the use of surfactants. The normal cascade depends strongly on the amount of time it takes for the air layer between the drop and pool to drain. By making the pool a liquid with a much greater surface tension value than the drop, the researchers sped up the air layer’s drainage. The mismatch in surface tension between the drop and pool creates an outward flow on the surface (below) due to the Marangoni effect. As the pool’s liquid moves outward, it drags air with it, thereby draining the separating layer more quickly. The result is still a coalescence cascade but one in which the later stages have no rebound and coalesce quickly. (Image and research credit: S. Shim and H. Stone, source)


The Colorful Dissolution of Candies
Many solids can dissolve in liquids like water, and while this is often treated as a matter of chemistry, fluid dynamics can play a role as well. As seen in this video by Beauty of Science, the dissolving candy coating of an M&M spreads outward from the candy. This is likely surface-tension-driven; as the coating dissolves, it changes the surface tension near the candy and flow starts moving away thanks to the Marangoni effect. With multiple candies dissolving near one another, these outward flows interfere and create more complex flow patterns.
These flows directly affect the dissolving process by altering flow near the candy surface, which may increase the rate of dissolution by scouring away loose coating. They can also change the concentration of dissolved coating in different areas, which then feeds back to the flow by changing the surface tension gradient. (Video and image credit: Beauty of Science)

Self-Propelling Drops
Droplets of acetone deposited on a bath of warm water can float along on a Leidenfrost-like vapor layer. The droplets are self-propelling, too, thanks to interactions between the acetone and water. Acetone can dissolve in water, and when acetone vapor beneath the drop gets absorbed into the water bath, it lowers the local surface tension. That drop in surface tension creates a pull in the direction of a higher surface tension; this is what is known as the Marangoni effect. Because of that flow in the direction of higher surface tension, the acetone drop accelerates away. (Image credit: S. Janssens et al., source)

Bursting Droplets
Mixing multiple fluids can often lead to surprising and mesmerizing effects, whether it’s droplets that dance or tears along the walls of a wine glass. A recent paper highlights another such mixture-driven instability – the bursting of a water-alcohol droplet deposited on an oil bath. The Lutetium Project tackles the physics behind this colorful burst in the short video above. The behavior is driven by the quick evaporation rate of alcohol in the droplet and the way this changing chemical concentration affects surface tension in the droplet. Alcohol evaporates more quickly from the edges of the drop, creating a region of higher surface tension around the edge. This pulls fluid to the rim of the drop, where it breaks up into droplets that get pulled outward as the inner drop shrinks.
The oil bath plays an important role in the instability, too. Without it, friction between the drop and a wall is too high for the droplet to “burst”. A thick layer of oil acts as a lubricant, allowing the escaping satellite drops to speed away. (Video and image credit: The Lutetium Project; research credit: L. Keiser et al.; submitted by G. Durey)


Four Seasons
The team behind Beauty of Science decided to explore the four seasons in this video combining macro footage of crystal growth, chemical reactions, and fluid dynamics. It’s always a fun game with videos like this to try and guess exactly what makes the mesmerizing patterns we see. Are those blue streaming waves in Spring caused by alcohol shifting the surface tension in a mixture? Are the dots of color welling up in Autumn a lighter fluid bursting up from underneath a denser one? As fun as the visuals are, though, what really made this video stand out for me was its excellent use of “The Blue Danube” to tie everything together. Check it out and don’t forget the audio! (Video credit: Beauty of Science; via Gizmodo)





Soap Bubbles Up Close
Watching soap bubbles up close is endlessly fascinating. The iridescent colors reflect the soap film’s thickness, or, in the case of black spots, its lack thereof. The dancing of the colors shows the soap film’s flow and the ever-shifting balance of surface tension necessary to keep the film intact. Even the junctures of the bubbles–so precise and mathematically perfect in structure–reflect the molecular interactions that govern them. (Video credit: Stereokroma; via R. Weston)

Dissolving
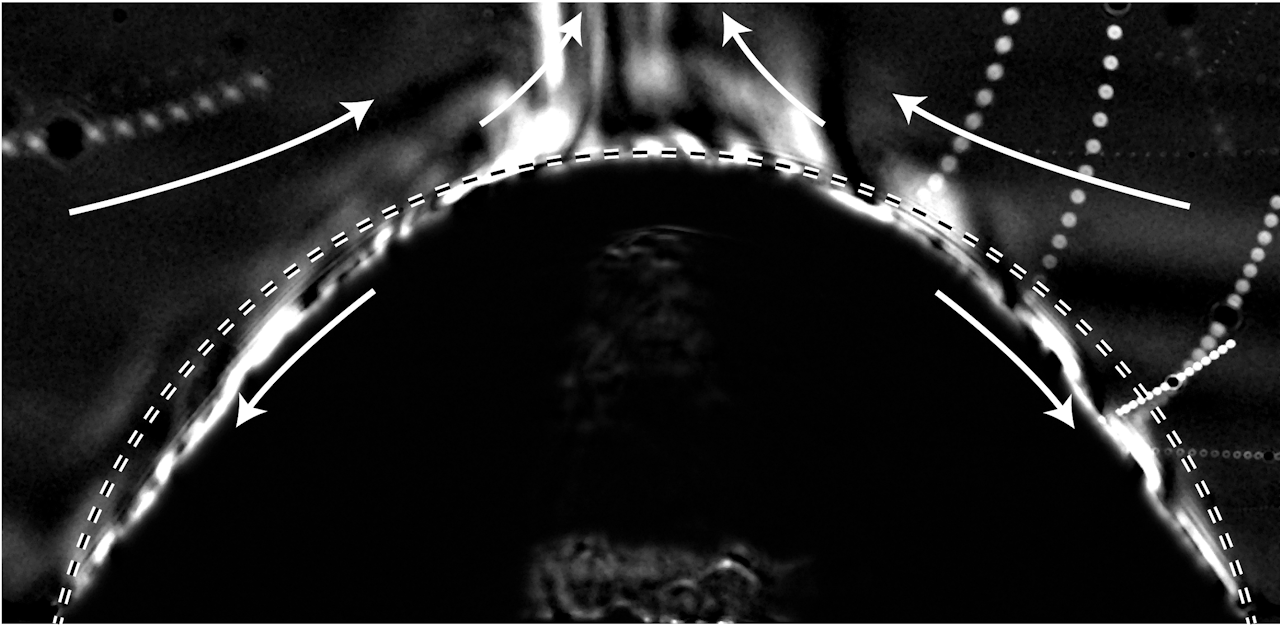
It looks like the fiery edge of a star’s corona, but this photo actually shows a dissolving droplet. The droplet, shown as the lower dark region in this shadowgraph image, is a mixture of pentanol and decanol sitting in a bath of water. Pentanol is a type of alcohol that is fully miscible with decanol and is water soluble, so that it will dissolve into the surrounding water over time. Decanol, on the other hand, is immiscible with water, so that part of the droplet won’t mix with the surrounding water.
The bright swirls along the droplet’s edge show areas with more pentanol. As the alcohol dissolves into the water, it forms a buoyant plume at the top of the droplet that rises due to pentanol’s lower density. That rising plume draws fresh water in from the sides, shown by the upper white arrows. Inside the droplet, flow moves in the opposite direction, from the top toward the outer edges. This is a result of uneven surface tension within the droplet. Scientists are interested in understanding the dynamics of these multiple component drops for applications like printing, where it’s desirable for pigments in an ink drop to be distributed evenly as the drop dries. (Image credit: E. Dietrich et al.)

Ink Drops Spreading
Ink drops atop a layer of glycerol spread in a beautiful fan of blue and white. The ink’s motion is the result of two processes: molecular diffusion and the Marangoni effect. Molecular diffusion is the mixing that occurs due to the random background motion of molecules. Since glycerol is a very viscous liquid, the ink is quite slow to spread in this manner.
The second factor, the Marangoni effect, is driven by differences in surface tension. The ink and glycerol have different surface tensions, and the exact values depend on concentration. Notice how the ink drops spread fastest from areas where the ink is densely concentrated. This tells us that the ink’s surface tension is lower than the glycerol’s. As a result, the glycerol’s higher surface tension tends to pull ink toward it. As the ink spreads and its concentration decreases relative to the glycerol, the ink-glycerol mixture’s surface tension increases. Since the difference between the surface tension of the mixture and the pure glycerol is not as large, the Marangoni force is reduced and the spreading slows. (Image credit: C. Kalelkar, source)