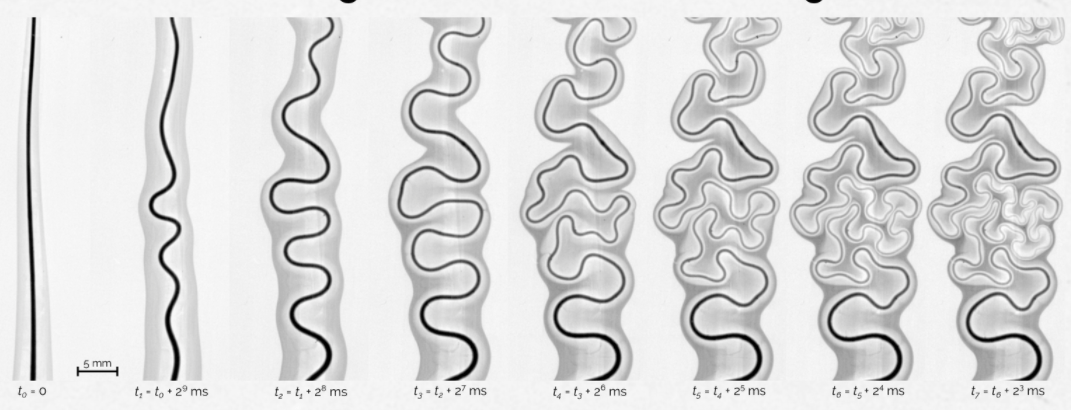
A thread of mineral oil laid across a pool of water twists and turns like a river run wild. Because the oil has a lower surface tension than the water, Marangoni forces spread it outward (far left). Small variations in the thread make the areas of highest oil concentration start to bend just a bit. Inside the bends, the gradient of surface tension – the difference between the lowest and highest surface tensions – is very high, which pulls at these regions more than others. So bends beget more bends, causing the entire thread to wrinkle. Although the behavior is driven by a completely different process than the one that causes rivers to meander, the end result looks remarkably similar; this is because, in both cases, forces act to make each bend increasingly sinuous. (Image credit: B. Néel et al., source)
Editor’s note: Starting tomorrow I’ll be on a trip that takes me out of range of the Internet until next week. Regular posts are queued up and should post as usual, but we’ll all have to trust Tumblr to handle everything because I won’t be able to check. Thanks!