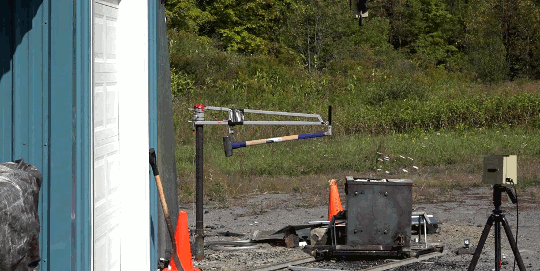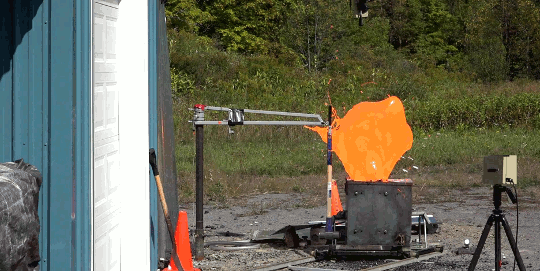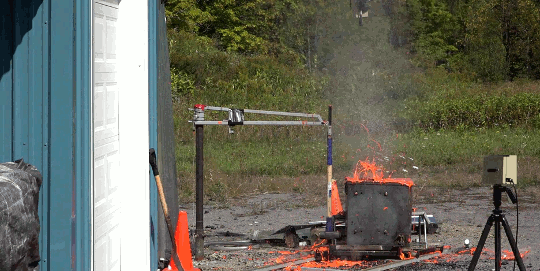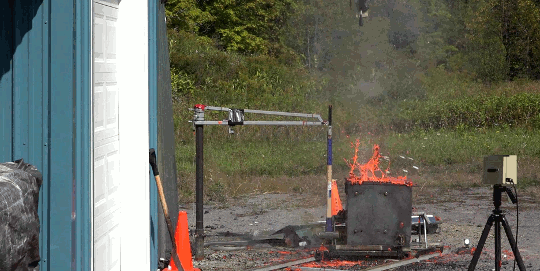
I’m just going to start this one with a blanket statement: DO NOT TRY THIS. Instead, enjoy the fact that the Internet enables us to enjoy the sight of burning gasoline in slow mo without any danger to ourselves.
In this video, Gav and Dan capture a burning bucket of gasoline as it’s thrown against glass. One thing this stunt really highlights is that it’s not the liquid gasoline that burns, it’s the vapor. However, since gasoline is volatile – in other words, it evaporates easily – the fire is quick to spread, especially as the toss atomizes droplets near the edge of the fluid. That’s why you see distinct streaks near the edge of the spreading flame and a non-burning liquid in the center. (Image and video credit: The Slow Mo Guys)