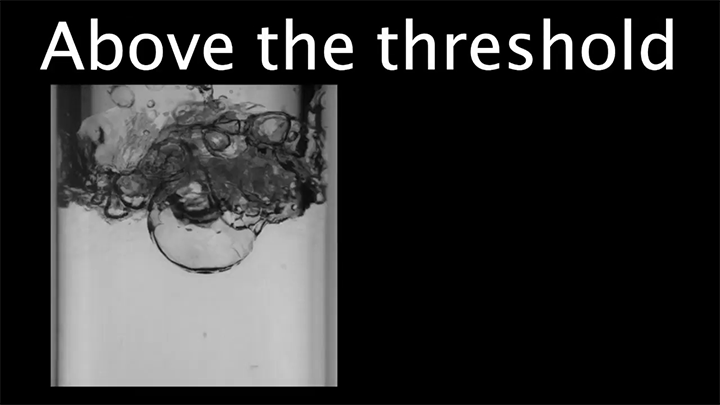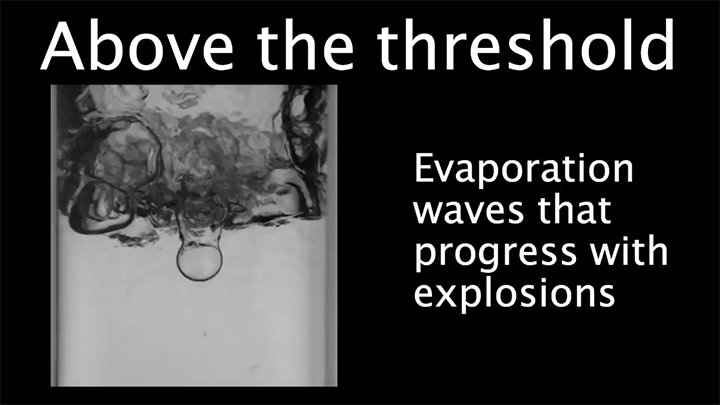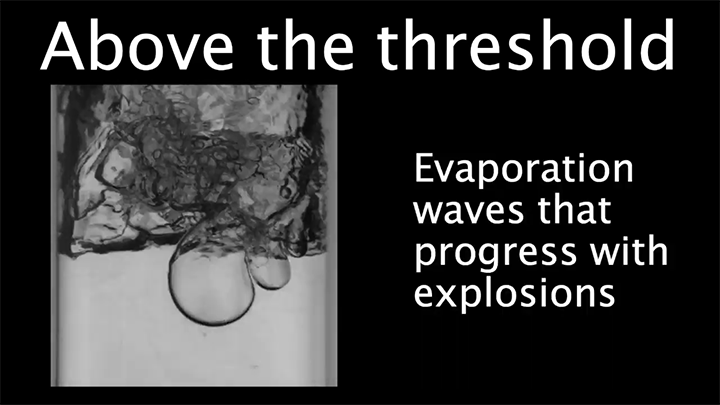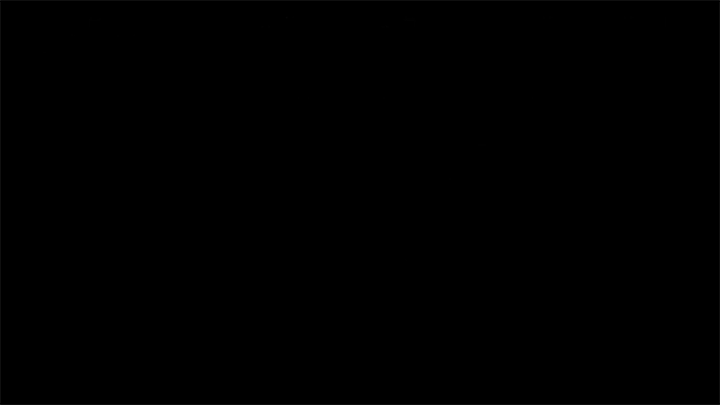
When pressurized, liquids can be superheated to temperatures well above their normal boiling point. When the pressure is released, the liquid will start boiling, sometimes explosively. In this video, researchers explore that dynamic by “racing” a series of liquids against one another. Each racer has been heated to a different temperature beyond the expected boiling point.
The clear winner is the liquid with the highest overheat; as explained in the latter part of the video, beyond a critical overheat temperature, vaporization waves in the fluid enhance the boiling, helping vaporization take place faster. (Video and image credit: K. Jing et al.)