Fluid dynamics and art have gone hand-in-hand for centuries. In this video, artist Garip Ay demonstrates one of the coolest fluids-based art techniques: paper marbling. In this technique, artists float ink or paints on a liquid surface, manipulate the colors as desired–in this case to recreate Van Gogh’s “Starry Night”–and then float a piece of paper atop the surface to transfer the image. Multiple cultures around the world developed marbling techniques, dating all the way back to the Middle Ages. Ay is an expert in ebru, a Turkish form of the art. For more of Ay’s art, check out his website and YouTube channel. (Video credit: G. Ay; via Gizmodo)
Tag: surfactant

Whiskey Stains
Photographer Ernie Button discovered that whiskey left behind intriguing patterns after it evaporated. Unlike coffee rings, the whiskey leaves behind a more uniform residue. Curious, he contacted researchers at Princeton, who were eventually able to explain why whiskey and coffee dry so differently. They observed three major effects in drying whiskey mixtures. Firstly, the alcohol in whiskey evaporates faster than other components, creating differences in concentration and, therefore, surface tension along the droplet. These variations in surface tension create Marangoni flow, which tends to mix the droplet. Coffee, being non-alcoholic, does not do this.
Whiskey also contains surfactants, low surface tension chemicals, which help pull particulates away from the edge of the droplet so they aren’t trapped there like in coffee. And finally, they found that the polymers in whiskey helped glue particles to the glass so that they were less likely to be carried by the flow. Taken together, these three ingredients – alcohol, surfactants, and polymers – all help make the whiskey stain more uniform. For more, watch the video below, see Button’s website, or check out the research paper. (Image credit: E. Button; research credit: H. Kim et al.; video credit: C&EN; submitted by @tommyjwilson)

Ode to Bubbles
Boiling water plays a major role in the steam cycles we use to generate power. One of the challenges in these systems is that it’s hard to control the rate of bubble formation when boiling. In this video, researchers demonstrate their new method for bubble control in a clever and amusing fashion. The twin keys to their success are surfactants and electricity. Surfactant molecules, like soap, have both a polar (hydrophilic) end and a non-polar (hydrophobic) end. By applying an electric field at the metal surface, the researchers can attract or repel surfactant molecules from the wall, making it either hydrophobic or hydrophilic depending on the field’s polarity. Since hydrophobic surfaces have a high rate of bubble formation, this lets the scientists essentially turn nucleation on and off with the flip of a switch! (Video credit: MIT Device Research Lab; see also: research paper, MIT News Video, press release)
Do you enjoy FYFD and want to help support it? Then please consider becoming a patron!

Draining Soap Film
The brilliant colors of a soap film are directly related to the film’s thickness. Black regions, like the one in the upper right of this image, are the thinnest regions and may be less than 100 nanometers thick. (That’s smaller than the shortest wavelength of visible light!) The colors of the peacock-feather-like blooms along the bottom of the image demonstrate significant variations in film thickness. This is caused by uneven concentrations of surfactants in the film. The variations in concentration causes differences in local surface tension, which in turn moves fluid around within the film. This is known as a Marangoni effect. (Image credit: S. Berg and S. Troian)

Glow-Stick Ferrofluids
Ferrofluids create all kinds of fascinating shapes when exposed to magnetic fields. In this video, Dianna from Physics Girl shows off what happens when you combine a ferrofluid with glowsticks and explains how ferrofluids get some of their unique properties. Ferrofluids consist of tiny nanoparticles of magnetic material that are surrounded by surfactants and suspended in a carrier fluid. This creates a fluid whose shape depends on gravity, surface tension, and the local magnetic field. By manipulating the relative strength of these forces, you can create everything from spikes to maze-like patterns to whatever this is. (Video credit and submission: Physics Girl)

Healing Soap Films
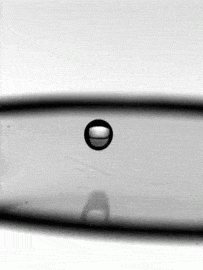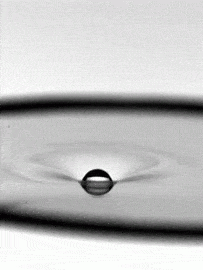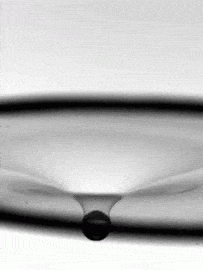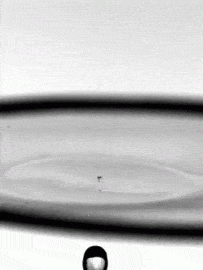
As fragile as a soap bubble seems, these films have remarkable powers of self-healing. The animation above shows a falling water droplet passing through a soap film without bursting it. An important factor here is that the water droplet is wet–passing a dry object through a soap film is a quick way to burst it, as those who have played with bubbles know. The droplet’s inertia deforms the soap film, creating a cavity. If the drop’s momentum were smaller, the film could actually bounce the droplet back like a trampoline, but here the droplet wins out. The film breaks enough to let the drop through, but its cavity quickly pinches off and the film heals thanks to the stabilizing effect of its soapy surfactants. (Image credit: H. Kim, source)

Plume Stratification
Clean-up of accidents like the 2010 Deepwater Horizon oil spill can be complicated by what goes on beneath the ocean surface. Variations in temperature and salinity in seawater create stratification, stacked layers of water with differing densities. When less dense layers are on top, the fluid is said to be stably stratified. Since oil is less dense than water, one might assume that buoyancy should make an oil plume should rise straight to the ocean surface. But the presence of additives or surfactants in the oil mixture plume can prevent that. With surfactants present, an oil mixture tends to emulsify, breaking into tiny droplets like a well-mixed salad dressing. Even if the density of the emulsion is smaller than the surrounding fluids, such a plume can get trapped at a density boundary, as seen in the photo above. Researchers report a critical escape height, which depending on the plume’s characteristics and stratification boundary, determines whether a plume escapes or becomes trapped. (Image credit: R. Camassa et al.)

Soap Film Physics
Soap films consist predominantly of water, yet their thin, virtually two-dimensional nature is impossible for water alone to achieve. The small amount of added soap acts as a surfactant, lowering the surface tension of the fluid and preventing it from bursting into droplets. When forming a film, the soap molecules align themselves along the outer surfaces of the film, with their hydrophilic heads among the water molecules and their hydrophobic tails oriented outward. For the most part, the water molecules stay sandwiched between the surfactant layers, forming a film only about as thick as the wavelength of visible light. In fact, the psychedelic colors of a soap film are directly related to the film’s thickness with the black regions being the thinnest. The video above shows a horizontal soap film at the microscopic scale and some of the dynamics exist therein. (Video credit: J. Hart)

Oily Foams
It is common in many industries to use oil as a defoamer to break up existing foams or prevent foams from forming. But with the right surfactants–additives that change the foam’s surface tension–it’s possible to make aqueous foams that are actually stabilized by the presence of oil. This video explores some of the ways that oil can interact with these kinds of foam, beginning with capillary action, which draws the oil up into the junctions between foam films. For more, see Piroird and Lorenceau. (Video credit and submission: K. Piroird)

Beads-on-a-string
Viscoelastic fluids are a type of non-Newtonian fluid in which the stress-strain relationship is time-dependent. They are often capable of generating normal stresses within the fluid that resist deformation, and this can lead to interesting behaviors like the bead-on-a-string instability shown above. In this phenomenon, a uniform filament of fluid develops into a series of large drops connected by thin filaments. Most fluids would simply break into droplets, but the normal stresses generated by the viscoelastic fluid prevent break-up. For this particular photo, the stresses are generated by clumps of surfactant molecules within the wormlike micellar fluid. Similar effects are observed in polymer-laced fluids. (Photo credit: M. Sostarecz and A. Belmonte)