In “Paradolia,” filmmaker Susi Sie plays with pareidolia, our tendency to seek patterns in nebulous data — like faces on a slice of toast. Droplets of miscible and immiscible fluids collide, part, and mix in each sequence, providing plenty of fodder for an active imagination. For myself, my brain especially likes assigning cartoon expressions to well-spaced drops in the video. What do you see? (Video and image credit: S. Sie)
Tag: miscibility

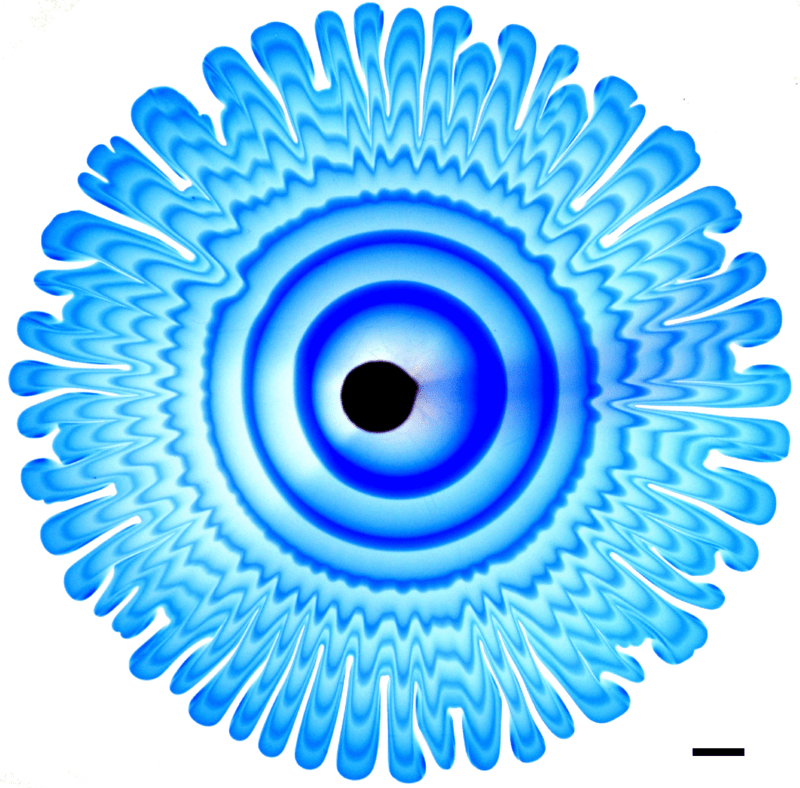
Peering Inside Viscous Fingering
Viscous fingers form when a low-viscosity fluid is pumped into a narrow, viscous-fluid-filled gap. The branching pattern that forms depends on the ratio of the two viscosities, among other factors. To better understand what goes on inside these fingers, researchers carefully alternated injecting dyed and undyed fluid. This creates a pattern of concentric rings that deform as the fingers spread.
In this particular study, the initial fluid and injected fluids are miscible, meaning that they can mix into one another. In modeling their experiments, the team found that this mixing created stratification — i.e., layers of fluids with different densities — in the narrow gap between their plates. The stratification’s effects were large enough that the model required a correction term for them; that’s a bit surprising because we’d usually expect that the tiny third-dimension of the gap would be too small to matter! (Image and research credit: S. Gowan et al.)

Billowing Ouzo
Pour the Greek liquor ouzo into water, and your glass will billow with a milky, white cloud, formed from tiny oil droplets. The drink’s unusual dynamics come from the interactions of three ingredients: water, oil, and ethanol. Ethanol is able to dissolve in both water and oil, but water and oil themselves do not mix.
In this video, researchers explore the turbulent effects of pouring ouzo into water. In particular, pouring from the top creates a fountain-like effect, due to a tug-of-war between the ouzo’s momentum and its buoyancy. Momentum wants the ouzo to push down into the water, and buoyancy tries to lift it back up. For an extra neat effect, they also show what happens when the ouzo is confined to a 2D plane and what happens when momentum and buoyancy act together instead of oppositely. (Image and video credit: Y. Lee et al.)

“Microscopic World”
So many natural processes take place right in front of us, but they’re too small and too fast to see. Here, the Beauty of Science team puts some of those processes — crystallizing solids, nucleating bubbles, and more — front and center. The shapes and colors draw you in, inviting you to engage with science we see daily but rarely appreciate. (Video and image credit: Beauty of Science)

Toying With Density and Miscibility
Steve Mould opens this video with a classic physics toy that uses materials of different densities as a brainteaser. Two transparent, immiscible liquids fill the container, along with beads of a couple different densities. When you shake the toy, the liquids emulsify, creating a layer with an intermediate density. As the two liquids separate, the emulsified middle layer disappears, causing the beads (which have densities between that of the two original liquids) to come together.
The rest of the video describes the challenges of expanding this set-up into three immiscible liquids and four sets of beads. Along the way, Steve had to contend with issues of miscibility, refractive index, and even chemical solvents. It’s amazing, sometimes, what it takes to make a seemingly simple idea into reality. (Video and image credit: S. Mould)

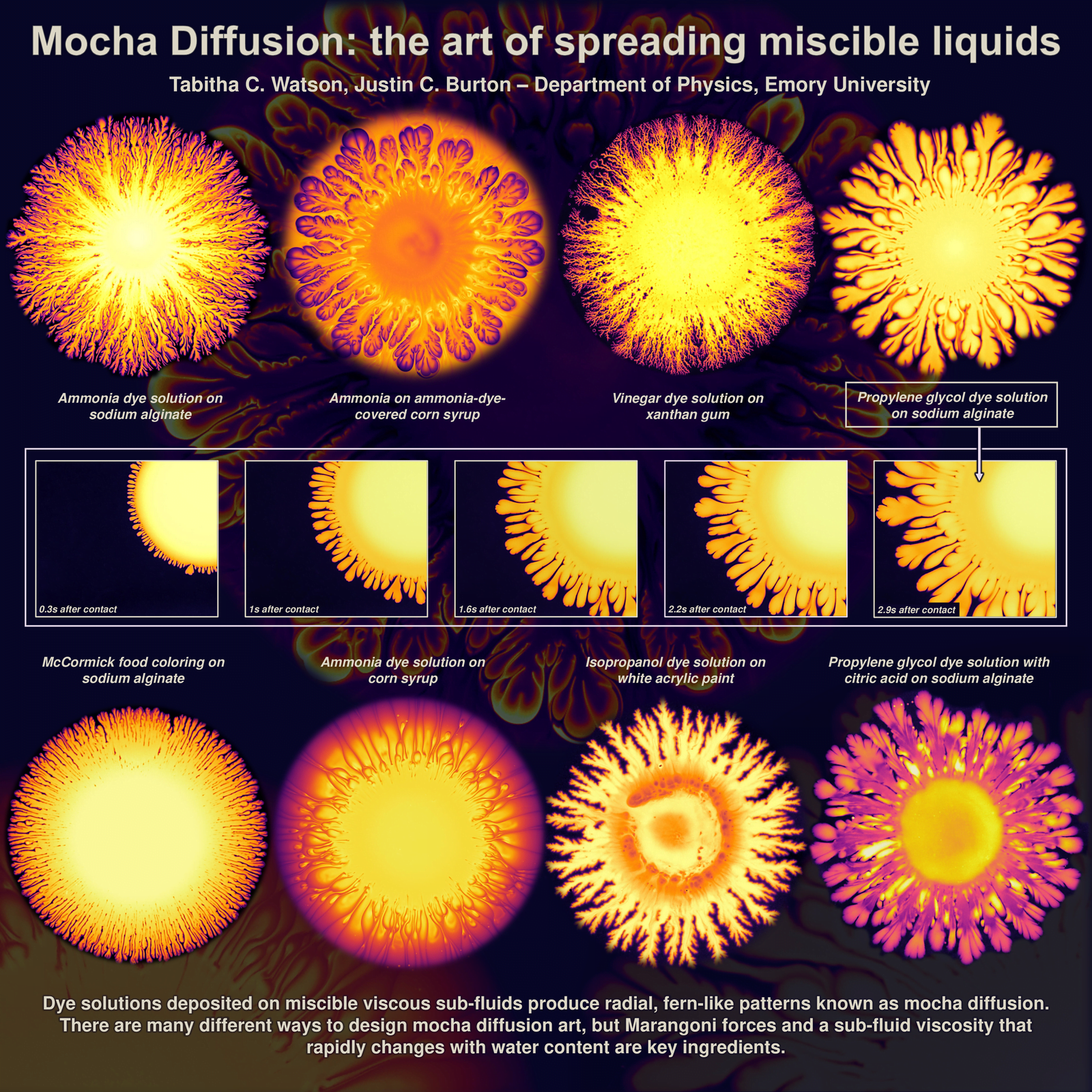
Mocha Diffusion
These firework-like patterns spread when dyes are added atop a viscous but miscible lower fluid layer. Here, researchers use lower layers like corn syrup and xanthan gum; then they spread dye mixtures including ammonia and vinegar atop those layers. Because the upper and lower layers of fluid are miscible and can diffuse into one another, they together form elaborate patterns. The mixing of the two layers creates gradients in surface tension that can drive the flow and create these mocha diffusion patterns. (Image credit: T. Watson and J. Burton)

“Bubbles Experience”
Acrylic paint, oil, water, and air combine to create ephemeral sculptures in Alberto Seveso’s “Bubbles Experience” series. I love the mixture of shapes he achieves, from large, seemingly-laminar columns to a mist of bubbles, each trailing a painted tail. They’re like tiny, liquid comets. See more from this series here and find more examples of his work in his online portfolio. (Image credit: A. Seveso)

“ColorLover”
“ColorLover,” a short film by artist Rus Khasanov, is a delightful liquid rainbow. The video’s ingredients seem to be ink, paint, oil, and a bit of superhydrophobic coating primed to reveal a heart. I love that latter touch; it’s a cool way to use regular materials in a way that some might assume involved digital effects! (Video credit: R. Khasanov)

“Halo”
Fluids create mesmerizing practical effects in this new experimental film from the Julia Set Lab. I love how the visuals mess with your sense of scale. Some of the sequences look like they could be a solar firestorm or disintegrating sea ice, though in reality the camera’s field of view is probably smaller than your palm. The filmmakers provide no information on the fluids they use, but I spy some hints of partially miscible ingredients, some chemical reactions, and plenty of Marangoni action. (Video and submission credit: S. Bocci/Julia Set Lab)

Swapping Emulsions
Chemically speaking, oil and water don’t mix. But with a little fluid mechanical effort, it’s possible to make them an emulsion — a mixture of oil droplets in water or water droplets in oil. Researchers in the Netherlands discovered that the viscosity of these emulsions depends critically on which of those mixtures you have.
To create their emulsions, the team used a tank consisting of two concentric cylinders. When the inner cylinder spins, it creates a well-understood flow field between the inner and outer cylinder. By varying the ratio of oil to water in the tank, they could explore a wide range of emulsions. They found that the emulsion’s viscosity changed dramatically when the emulsion shifted from oil droplets in water to water droplets in oil, something known as a catastrophic phase inversion. During this switch the viscosity dropped from 3 times higher than pure water to 2 times lower! (Image credit: A_Different_Perspective; research credit: D. Bakhuis et al.; via APS Physics; submitted by Kam-Yung Soh)