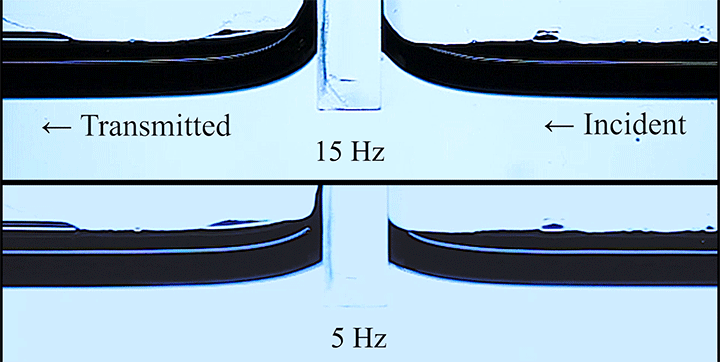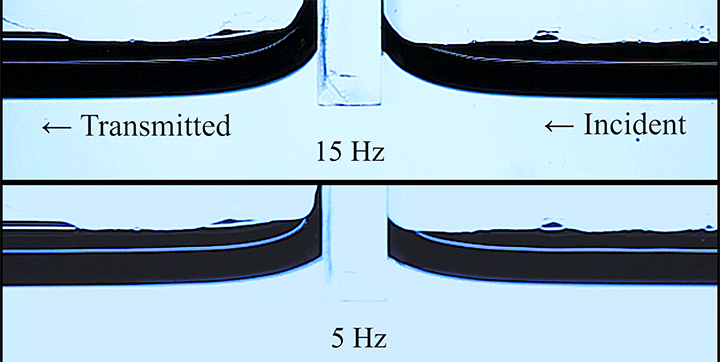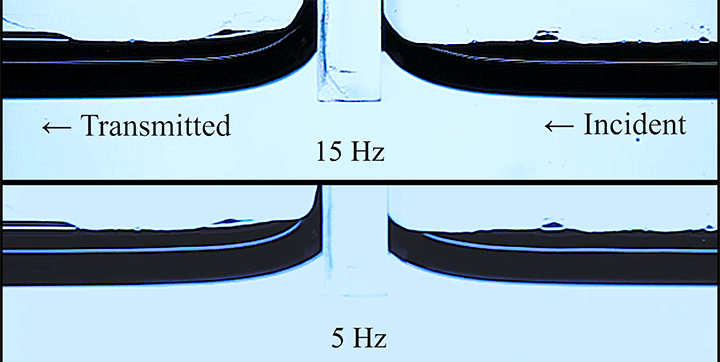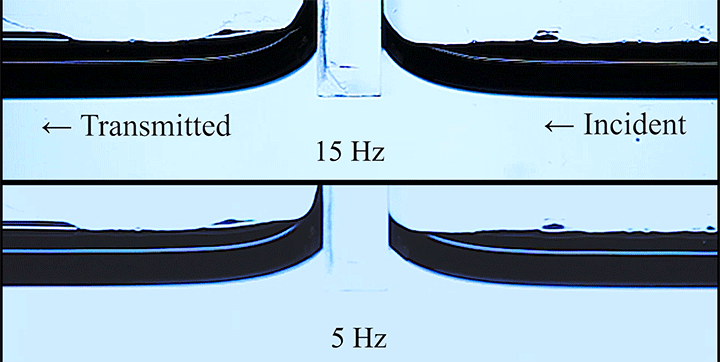
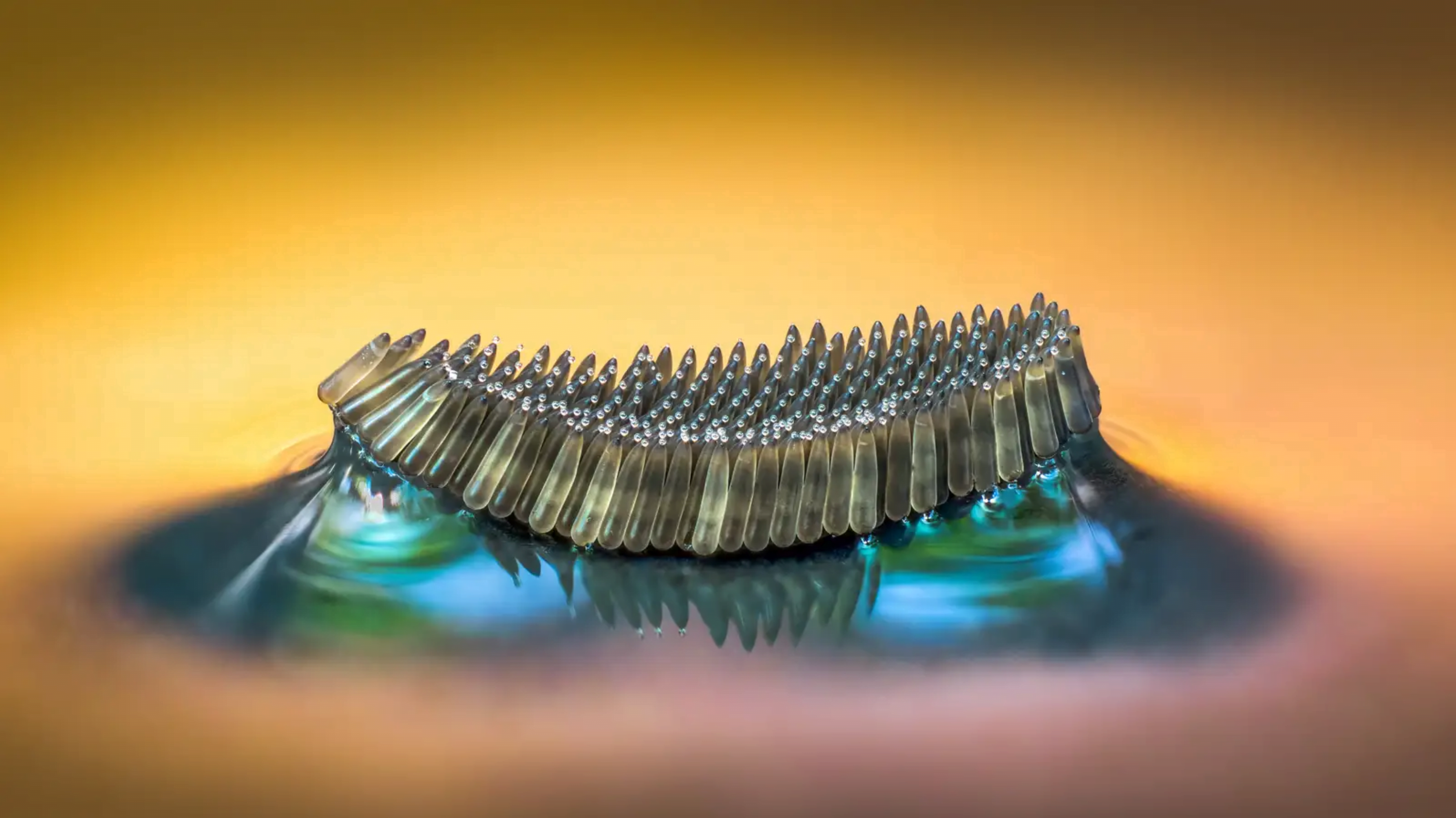
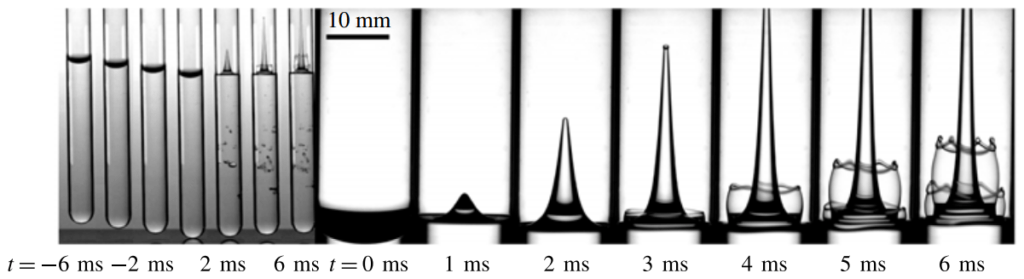
Even small changes to a meniscus can change how much wave energy passes through it. A new study systemically tests how meniscus size and shape affects the transmission of incoming waves.
As seen above, the meniscus was formed on a suspended barrier. By changing the barrier size and wettability as well as the characteristics of incoming waves, researchers were able to map out how the meniscus affected waves that made it past the barrier.
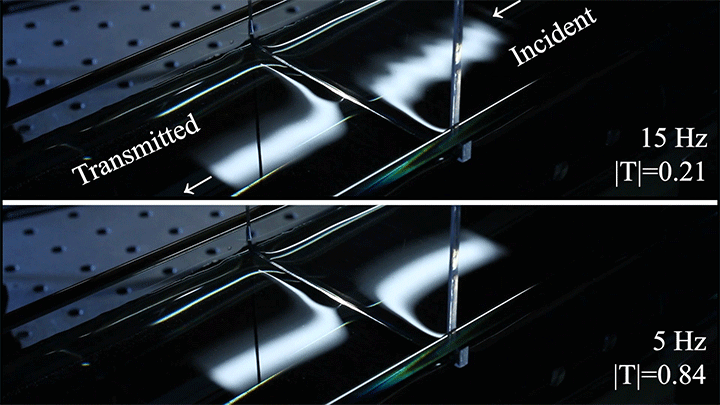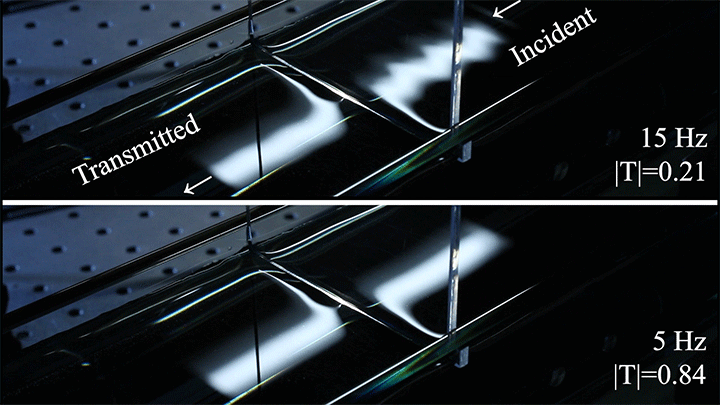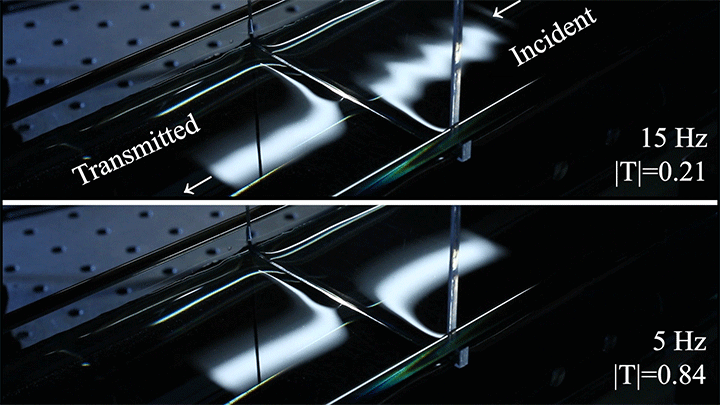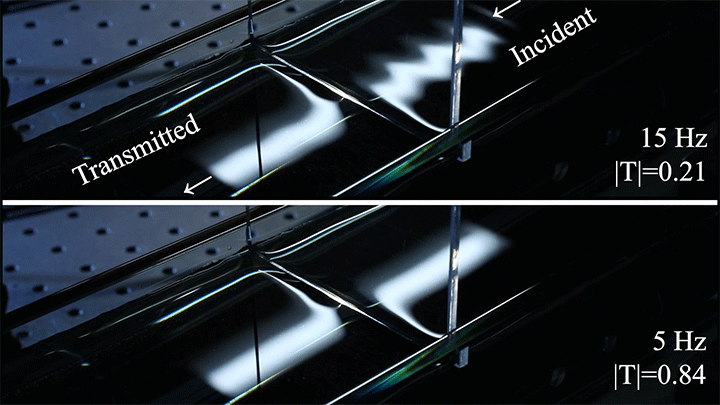
In particular, they found that drawing the meniscus upward by raising the barrier would, at first, enhance wave transmission but then suppressed wave energy as the barrier moved higher. They attributed the change in behavior to an interplay between water column height and meniscus inclination. (Research and image credit: Z. Wang et al.; via Physics World)