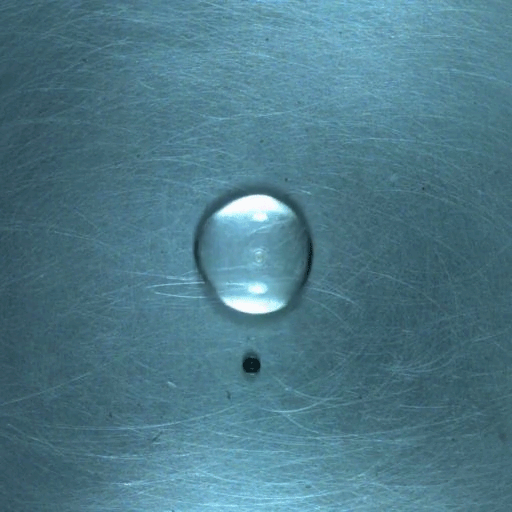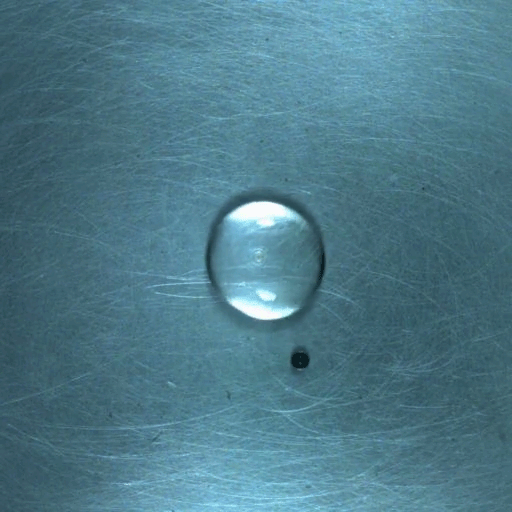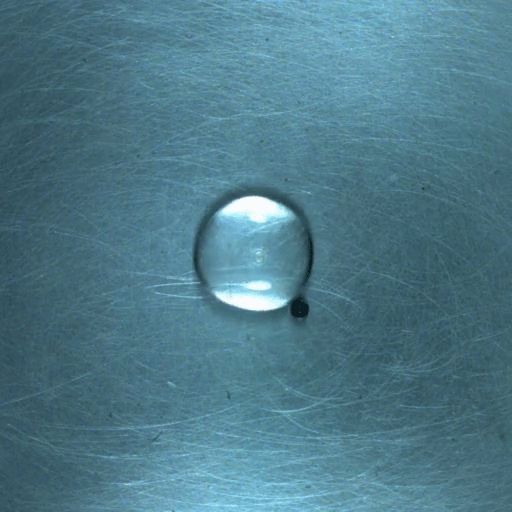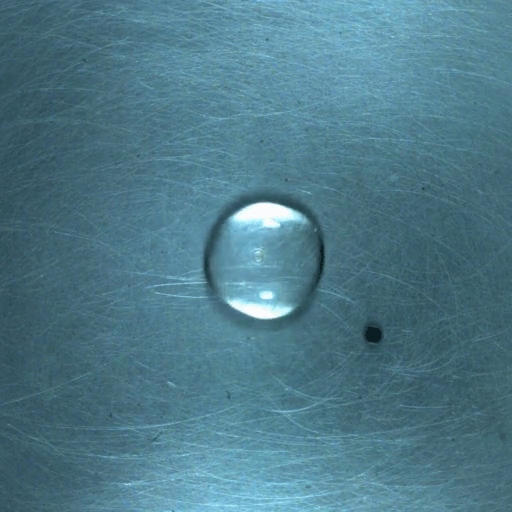
When a droplet encounters a surface much hotter than its boiling point, it forms a thin layer of vapor that insulates the liquid from the surface. But this Leidenfrost effect can’t last forever. Eventually, the vapor layer destabilizes and the drop touches the surface, causing explosive boiling that destroys the drop.
To determine how the layer destabilizes, researchers simulated the breakdown. To their surprise, they found that inertial forces in the micron-thin vapor layer were critical for destabilization. The gas inertia caused reductions in pressure that pulled the liquid toward the surface. Usually at these small scales, we’d ignore inertial effects and focus instead on viscosity, but, for Leidenfrost drops, that simplification doesn’t work. (Image credit: L. Gledhill; research credit: D. Harvey and J. Burton)