Ice stupas are conical artificial glaciers built with snow cannons; they’re used to store water for spring irrigation. Here, researchers explore a miniaturized lab-grown version made from atomized water droplets. The growing drop breaks and spills, forming frozen fingers in all directions. Further drops flow and freeze as rivulets atop the stupa — or they destabilize and rotate toward another finger, leaving behind a wrinkling shape. Although the formation works very differently (and the scales are completely different) these tiny ice stupas remind me of volcanic flows. (Image credit: D. Papa et al.)
Tag: ice formation

Ice Damages With Liquid Veins
Water expands when it freezes, a fact that’s often blamed for ice-cracked roads. But expansion isn’t what gives ice its destructive power. In fact, liquids that contract when freezing also break up materials like pavement and concrete. A recent study pinpoints veins between ice crystals as the source of this infrastructure-cracking power.
Ice doesn’t like to stick on most surfaces, so when it forms, there’s often a narrow gap between the ice and a solid surface. That gap fills with water, and that water, it turns out, doesn’t just sit there. Instead, grooves between ice crystals act like tiny straws that are frigid on the icy end and warmer on the end connected to water. As ice forms on the cold end, it creates a negative pressure gradient that draws liquid up the groove. This ‘cryosuction’ keeps pumping water into the ice, where it freezes and further expands the icy zone, as seen in the image below.

Under a microscope, fluorescent particles show water (right side) getting pulled into an ice groove (left). If the ice is made up of a single crystal, this growth rate is very slow. But most ice is polycrystalline — made up of many crystals, all separated by these liquid-filled grooves. That, researchers found, is a recipe for fast growth and quickly-expanding ice capable of breaking concrete and other structures. (Image credits: pothole – I. Taylor, experiment – D. Gerber et al.; research credit: D. Gerber et al.; via APS Physics)

Frozen Ripples
Normally, freezing is a slow enough process that transient phenomena like ripples get smoothed out. But with the right conditions, even ripples can get frozen in time. This picture shows a backyard bird bath after a frigid winter storm passed overnight. For much of that time, the wind was active enough to keep the bath’s water from freezing. But when freezing did start, it happened so rapidly that the wavelets generated by the wind got frozen in place, too. Here’s a similar-looking effect (also in Colorado, ironically) that’s thought to have formed entirely differently. (Image credit: K. Farrell; via EPOD; submitted by Kam-Yung Soh)

Spiral Ice Cracks
This odd puddle was found in Arizona after a night with low temperatures around -8 degrees Celsius (18 degrees Fahrenheit). Unlike the concentric rings sometimes seen on ice, this puddle formed one spiraling crack. It’s hard to know exactly what factors played into this formation since it was only found after the fact, but one possibility is that the puddle was initially frozen in a continuous sheet. Then, as the temperature cooled overnight, the ice contracted, forming a crack. As the ice kept cooling and contracting inward, the crack grew, spiraling toward the center of the puddle. (Image credit: M. Hendrickson; via EPOD; submitted by Kam-Yung Soh)

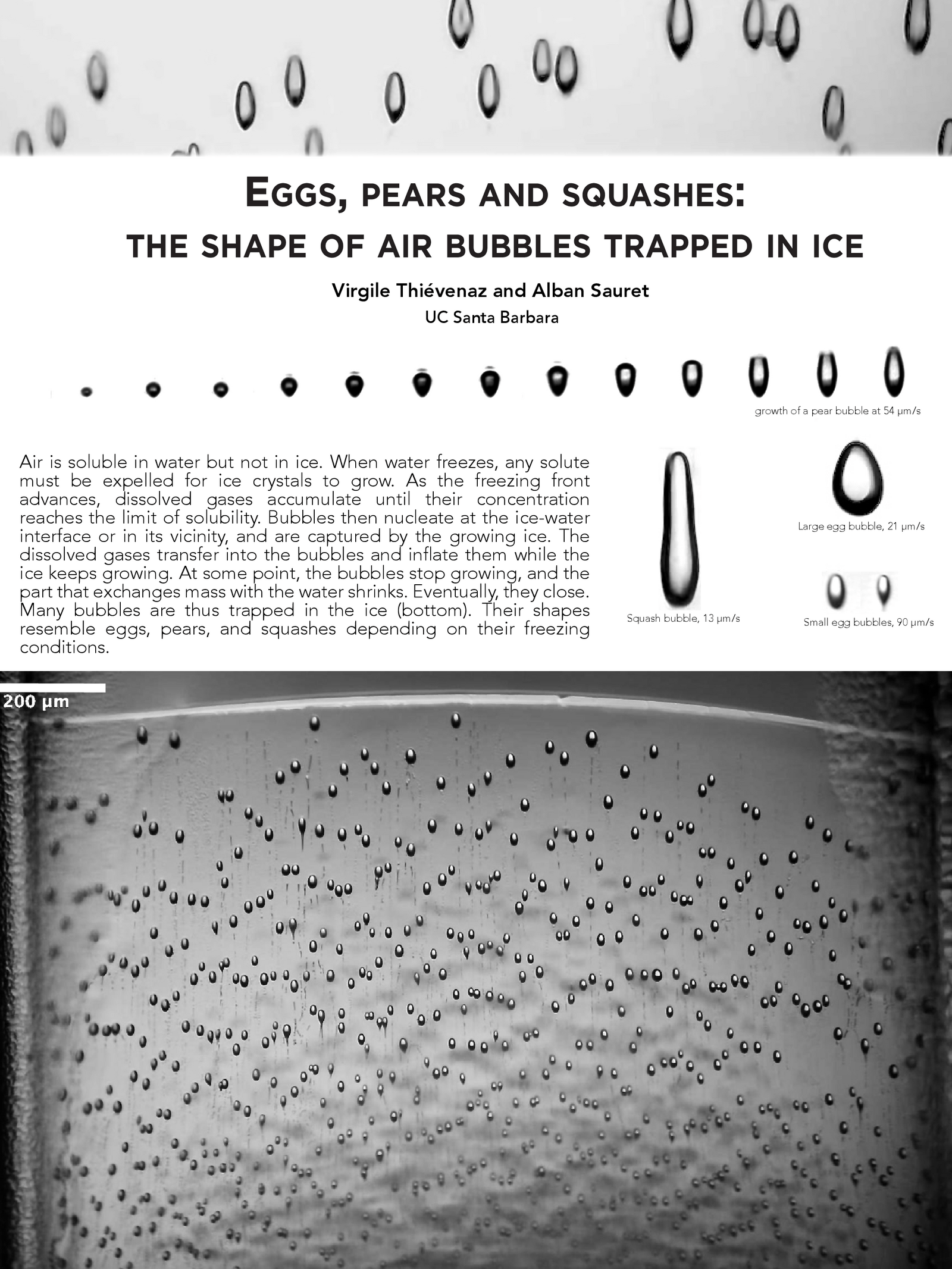
Frozen in Ice
Air can dissolve in water, but not in ice. So as water freezes, any dissolved gases have to get squeezed out in order for the ice crystals to grow. Once the concentration of gases is high enough, a bubble nucleates and gets captured by the growing ice around it. The shape of the final bubble depends on its freezing conditions. As seen here, bubbles take on all kinds of shapes, ranging from egg-like to a long and skinny squash-like shape. (Image credit: V. Thiévenaz and A. Sauret)

Predicting Alien Ice
Europa is an ocean world trapped beneath an ice shell tens of kilometers thick. To better understand what we might find in those oceans, researchers turn to analogs here on Earth, looking at Antarctica’s ice shelves. Beneath those shelves, ice forms via two mechanisms: the first, congelation ice, freezes directly onto the existing ice-water interface. The second, frazil ice, forms crystals in supercooled water columns, which drift upward in buoyant currents and settle on the ice shelf like upside-down snow (pictured above).
Based on Europa’s conditions, the researchers conclude that congelation ice would gradually thicken the ice shell as the moon’s interior cools. But in areas where the shell is thinned by local rifts and Jovian tidal forces, frazil ice is likely to form. (Image credit: H. Glazer; research credit: N. Wolfenbarger et al.; via Physics World)

Hydrophobic Ice
Water is an endlessly peculiar substance, eager to adopt many configurations. Each molecule can form up to four, highly-directional bonds. In this study, researchers found an unexpected configuration, a 2D type of ice known as bilayer hexagonal ice, on a corrugated gold surface. Bilayer hexagonal ice has been known since the late 1990s, but it was thought to be comparatively rare. In this form, water molecules assemble in an ice only two molecular layers thick, with hydrogen bonds between neighboring molecules taking up nearly all possible binding sites. With nowhere to bind, additional water cannot add to the ice’s thickness, making the ice as a whole hydrophobic or “water-fearing”.

This illustration shows a type of 2D ice, known as bilayer hexagonal ice, as it forms on a corrugated gold surface. From above (top half), the water molecules align to the surface with some molecules (red) in the troughs and others (pink) along the ridges. Viewed from the side (lower half), most of the molecules bind with their neighbors, leaving few H-bond sites available where more water layers of water could attach. This inability to add more vertical layers is why the ice appears hydrophobic. Previously, this type of ice had only been found on hydrophobic, flat surfaces. In the latest research, though, researchers found that surface corrugations allowed the ice to form, even on a surface that was only slightly hydrophobic. Observations like these help theorists modeling water and its interactions with surface. (Image credit: top – E. McKenna, illustration – APS/A. Stonebraker; research credit: P. Yang et. al.; via APS Physics; submitted by Kam-Yung Soh)

Erie Ice
Lake Erie, the shallowest of the Great Lakes, sees large swings in ice cover over the winter. In late January 2022, the lake was nearly completely frozen over, with 94 percent of its area covered in ice. By February 3rd, ice cover had dropped to 62 percent before rising again to 90 percent by the 5th. Air temperature and wind are the primary drivers of Erie’s fast ice growth and decay. As storms roll through, the ice can spread rapidly, but once temperatures rise, it takes very little forcing from the wind for the ice to begin breaking up. (Image credit: J. Stevens/USGS; via NASA Earth Observatory)

Jumping Frost
Liquid water is easily electrically charged, due to its polar nature. That’s why rubbing a comb is enough to deflect a stream of water. Ice is harder to charge, but it can happen, especially when there are temperature gradients across the ice.
That’s the key behind this study of jumping frost. When ice crystals grow on a surface much colder than their surroundings, positive charges gather in the colder region, leaving the dendritic branches of the ice negatively charged. When researchers brought liquid water near the charged ice crystals, the water became charged, too. Positive charges in the water attracted the negatively-charged dendrites, causing the ice crystals to jump off the surface.
Studies like this help us better understand cloud and rain formation and may one day lead to new ways of de-icing surfaces. (Image credit: frost – Miriams-Fotos, figure – R. Mukherjee et al.; research credit: R. Mukherjee et al.; via ChemBites; submitted by Kam-Yung Soh)


Rings of Ice
Heavy rains followed by a sudden freeze can produce icy puddles like this one. Because the pool was shallow to begin with, it likely froze rapidly. As the temperature continued dropping, the newly-formed ice contracted; the ring pattern of the cracks tells us the stress in the ice was primarily radial. Once formed, the cracks provided a path for any unfrozen water still in the puddle to get squeezed up onto the surface through capillary action and any further expansion or contraction of the ice. (Image credit: D. Stith; via EPOD; submitted by Kam-Yung Soh)