We’re surrounded daily by convection — a buoyancy-driven flow — but most of the time it’s invisible to us. In this video, Steve Mould shows off what convection really looks like with some of his excellent tabletop demos. The first half of the video gives profile views of turbulent convection, with chaotic and unsteady patterns. When he switches to oil instead of water, the higher viscosity (and lower Reynolds number) offer a more structured, laminar look. And finally, he shows a little non-temperature-dependent convection with a mixture of Tia Maria and cream, which convects due to evaporation changing the density. (Image and video credit: S. Mould; submitted by Eric W.)
Tag: buoyancy

Dancing Peanuts
Bartenders in Argentina sometimes entertain patrons by tossing a few peanuts into their beer. Initially, the peanuts sink, but after a few seconds they rise, wreathed in bubbles. Once on the surface, they roll, causing the bubbles to pop, and the peanut sinks once again. The cycle repeats, sometimes for as long as a couple hours.
There are a couple physical processes governing this dance. The first is bubble nucleation. Most beers are carbonated; they contain dissolved carbon dioxide gas that remains in solution while the beer is under pressure. Once poured, that storage pressure is gone and bubbles start to form in the liquid. The shape of the peanut means that bubbles form more easily on it than on the glass walls or in the liquid. And once the peanut is covered in bubbles, buoyancy comes into play. The bubbles attached to the peanut reduce its density relative to the surrounding fluid, enabling the peanut to rise up and float.
This same process is seen with other objects in carbonated fluids, too, such as blueberries in beer and lemon seeds in carbonated water. But it’s also reflected elsewhere in nature. For example, magnetite crystals are thought to float in magma due to a similar nucleation of dissolved gases on their surface. (Image and research credit: L. Pereira et al.; via APS Physics)

Bubble Cleaning
Removing dirt and bacteria from fruits and vegetables is a delicate job; too much force can bruise the produce and hasten spoiling. That’s why fluid mechanicians want to give the job to bubbles. Placing objects in a stream of air bubbles inside a bath is a surprisingly effective method for gently cleaning surfaces. A recent study finds that 22.5 degrees is the optimal angle for sliding bubbles to scrape a surface clean.
As the bubbles slide past the surface, they exert a shear force that scrapes away debris, just as you might use a loofah in the shower. The angle the bubble makes with the surface determines how long it’s in contact and how much force the bubble exerts. Increasing the angle makes the bubble slide faster, increasing its shear force. But above 22.5 degrees, the bubble’s buoyancy means that it spends less time pressed against the surface, which decreases its cleaning ability.
The team hopes to use their results to build a “fruit Jacuzzi” device that will direct bubble streams to gently and effectively clean fruits and vegetables in a matter of minutes. (Image and research credit: A. Hooshanginejad et al.; via APS Physics)

“Iridescent”
Soft colors and sudden coalescence combine in this short film from Susi Sie’s team. The visuals rely on liquid lenses (likely oil) floating atop a water bath. You can see how the fluids get manipulated in their behind-the-scenes video, which also provides a peek at how the sound effects get made. (Video credit: S. Sie et al.)

Airflow in the Opera
Like so many other performers, the singers and musicians of New York’s Metropolitan Opera House were left without a way to safely perform when the SARS-CoV-2 pandemic began in early 2020. In search of safe ways to perform and rehearse, the Met turned to researchers at nearby Princeton University, who worked directly with the performers to explore aerosol production and airflow in the context of professional opera.
Through visualization and other experiments, the team found that the highly-controlled breathing of opera singers actually posed a lower risk for spreading pathogens than typical speaking and breathing. Most of a singer’s voiced sounds are sustained vowels, which produce a slow, buoyant jet that remains close to a singer. The exception are consonants, which created rapid, forward-projected jets.
In the orchestra, the researchers found that placing a mask over the bell of wind instruments like the trombone reduced the speed and spread of air. One of the highest risk instruments they found was the oboe. Playing the oboe requires a long, slow release of air, but between musical phrases, oboists rapidly exhale any remaining air from their lungs and take a fresh breath. That rapid exhale creates a fast, forceful jet of air that necessitates placing the oboist further from others. (Image credit: top – P. Chiabrando, others – P. Bourrianne et al.; research credit: P. Bourrianne et al.; via APS Physics; submitted by Kam-Yung Soh)

“Bubbles Experience”
Acrylic paint, oil, water, and air combine to create ephemeral sculptures in Alberto Seveso’s “Bubbles Experience” series. I love the mixture of shapes he achieves, from large, seemingly-laminar columns to a mist of bubbles, each trailing a painted tail. They’re like tiny, liquid comets. See more from this series here and find more examples of his work in his online portfolio. (Image credit: A. Seveso)

Backswimmers
Backswimmers rule the surface of ponds, streams, and other bodies of water. These insects spend much of their time clinging just beneath the air-water interface, where they hunt larvae and other insects. They use oversized, oar-shaped back legs to row, and they breathe using an air bubble that clings to their abdomen like a personal scuba tank. Oxygen from the water diffuses into the bubble, keeping the insect’s air supply fresh. When the time comes to move to greener pastures, they flip to the other side of the water’s surface, unfurl their wings, and take off. (Image and video credit: Deep Look)

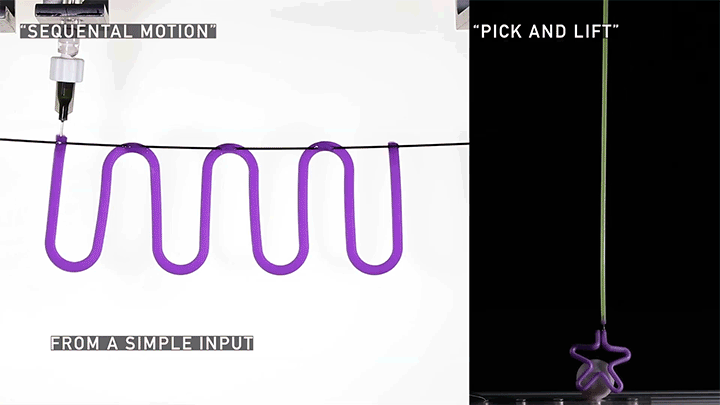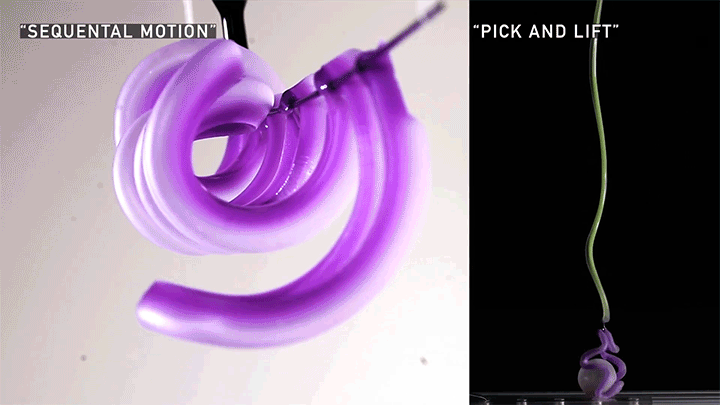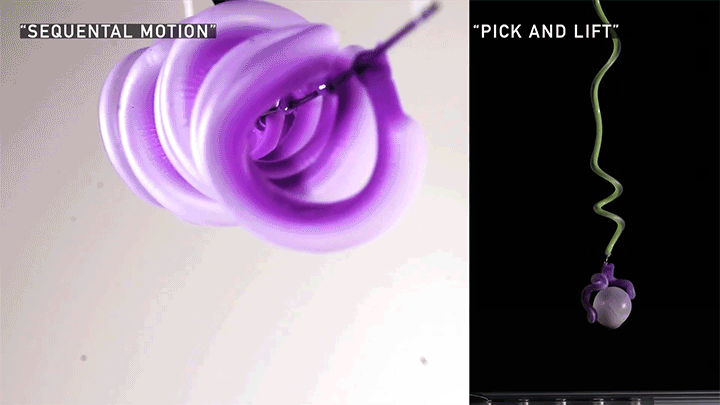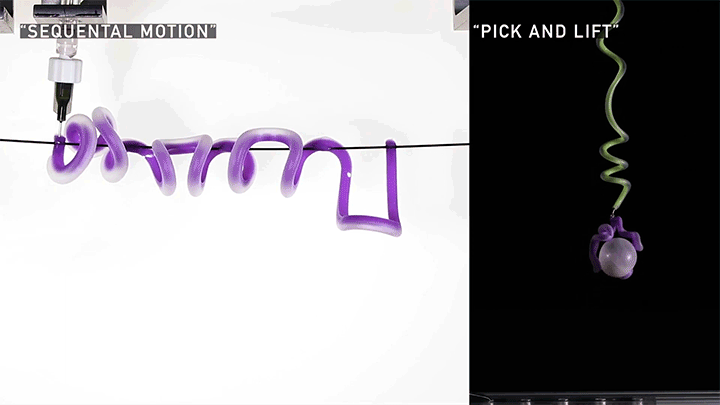
Squishy Actuators
Hard materials don’t always work well in robotics. Here, researchers build soft actuators that can bend, curl, and tighten in order to manipulate objects. They begin by injecting liquid elastomer into a tube (Image 1), followed by a bubble of air. Buoyancy makes the air bubble rise within the tube, creating an asymmetric cross-section where the solidified elastomer has a thin shell along one side and a thicker wall along the other (Image 2). When high-pressure air is pumped into the soft tubes, their asymmetric cross-section makes them bend and twist (Image 3). The team found that they can tune the elastomer tubes to form complex shapes good for gripping and flexing — perfect for a soft robot! (Video and image credit: T. Jones et al.; research credit: T. Jones et al.)

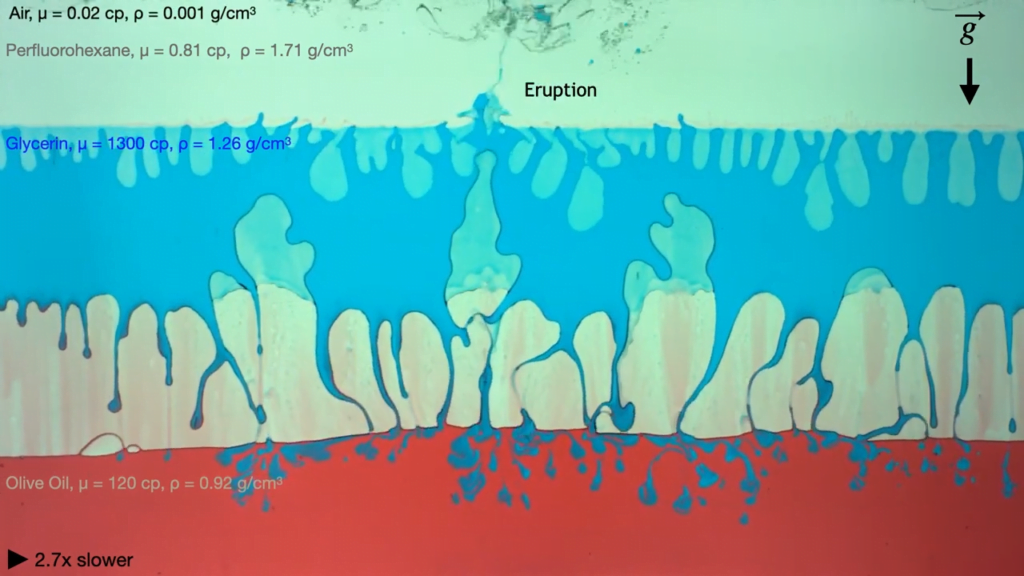
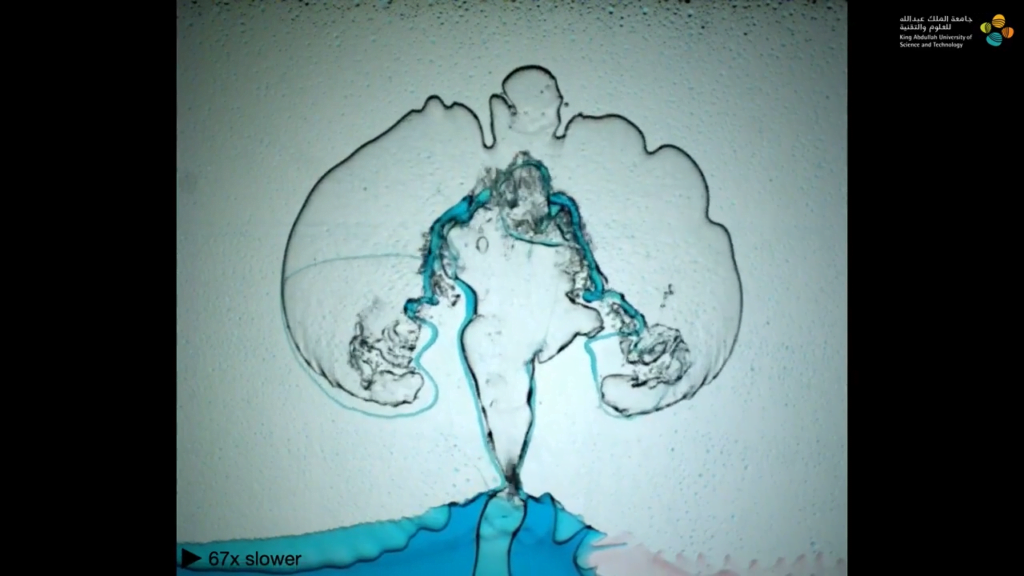
Eruption in a Box
In layers of viscous fluids, lighter and less viscous fluids can displace heavier, more viscous liquids. Here, researchers demonstrate this using four fluids sandwiched between layers of glass and mounted in a rotating frame. (Think of those liquid-air-sand art frames found in museums but bigger!)
In their first example, each layer of fluid is denser than the one beneath it, so buoyancy forces the lowest layer — air — to rise. The air pushes its way through the more viscous layer of olive oil, then slowly makes its way through the even more viscous glycerin before bursting through the last layer in an eruption. As the team varies the viscosity and miscibility of the layers, the movement of the buoyant fluids through the viscous layers changes dramatically. (Image and video credit: A. Albrahim et. al.)

When Bubbles Don’t Die
In a pure liquid, most bubbles pop almost immediately. But with a simple ingredient — a little heat — bubbles can live almost indefinitely. The mechanism is revealed in this video when the researchers use an infrared camera to watch a bubble on a heated pool. The top of the bubble is cooler than the rest of the liquid, forming colder, denser droplets that slide down. But the cooler liquid also has a higher surface tension, which draws warm liquid up the bubble, replenishing it. The result is a stable bubble that simply carries on. (Image and video credit: S. Nath et al.)