Giving droplets a kick by accelerating the surface they sit on creates elaborate shapes as the drops respond. As the surface accelerates upward, the droplet flattens into a pancake. When the plate slows down, the droplet continues rising, stretching into a cone as its rim flies upward and its lower surface adheres to the surface. The rim retracts with a constant acceleration while the drop detaches with a constant velocity. That velocity depends on how well it adheres to the surface. The interplay between those two variables determines how conical or cylindrical the drop appears. See more in the full video below. (Image and video credit: P. Chantelot et al.)
Tag: adhesion

Rain on Car Windows
As a child, I loved to ride in the car while it was raining. The raindrops on the window slid around in ways that fascinated and confused me. The idea that the raindrops ran up the window when the car moved made sense if the wind was pushing them, but why didn’t they just fly off instantly? I could not understand why they moved so slowly. I did not know it at the time, but this was my early introduction to boundary layers, the area of flow near a wall. Here, friction is a major force, causing the flow velocity to be zero at the wall and much faster – in this case roughly equal to the car’s speed – just a few millimeters away. This pushes different parts of large droplets unevenly. Notice how the thicker parts of the droplets move faster and more unsteadily than those right on the window. This is because the wind speed felt by the taller parts of the droplet is larger. Gravity and the water’s willingness to stick to the window surface help oppose the push of the wind, but at least with large drops at highway speeds, the wind’s force eventually wins out. (Image credit: A. Davidhazy, source; via Flow Viz)

Blowing Bubbles in Space
Blowing bubbles in your fruit juice is a bad idea when you’re in space, as astronaut Jack Fischer demonstrates. On Earth, gravity dominates water’s behavior, except when things are very small. But in microgravity, a liquid’s other characteristics become more obvious. Adhesion between the straw and juice guides it up and onto Fischer’s face. Surface tension is strong enough to hold the expanding juice bubble together. Capillary action, the ability of fluids to climb up narrow spaces, is far more apparent in microgravity as well, although it’s not important for this demo. We sometimes forget how powerful these forces can be, but microgravity is a good reminder that fluids are more complicated than we think. (Image credit: J. Fischer, source)

Easy Squeezing


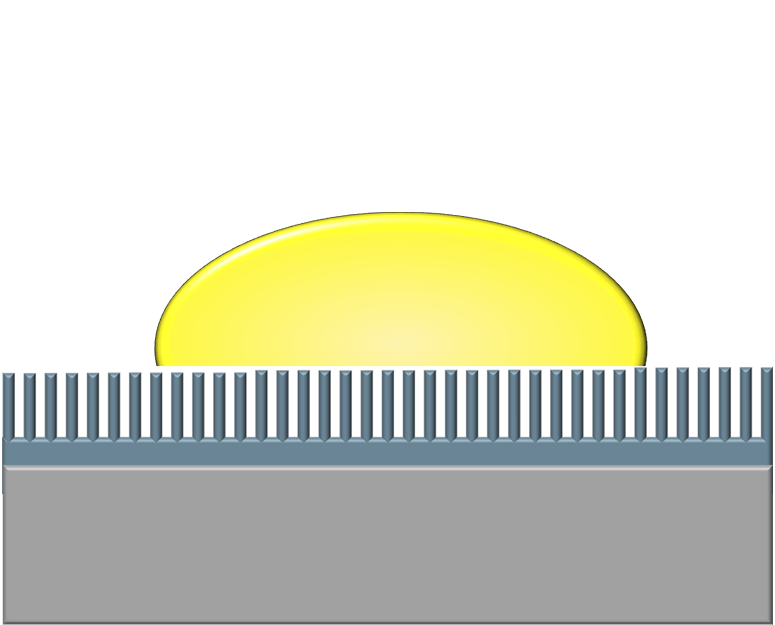
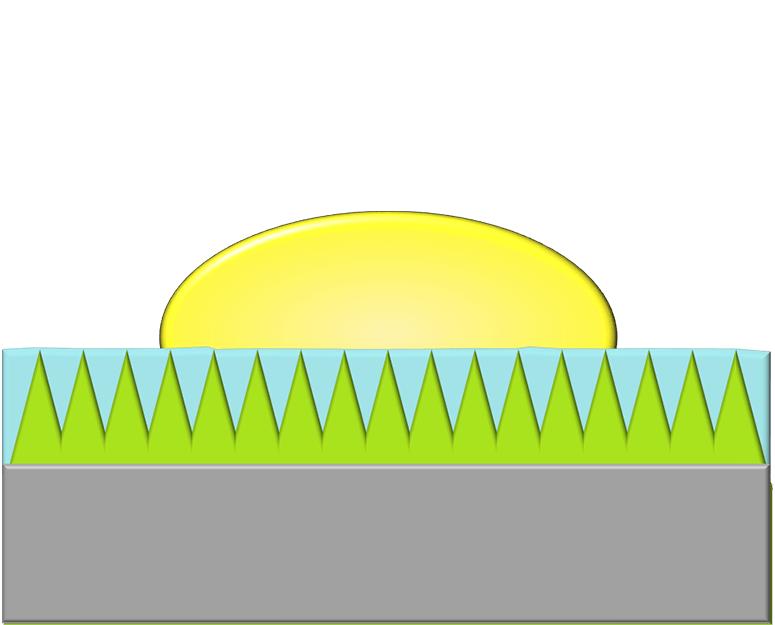
Nearly everyone has struggled with the frustration of trying to get ketchup, toothpaste, or peanut butter out of a container. These fluids and fluid-like substances are notoriously difficult to budge because they prefer to wet and adhere to solid surfaces. One way to limit this adhesion is to use a superhydrophobic surface, like the one shown in the middle image. These surfaces use micro- and nanoscale roughness to trap air pockets underneath a liquid and reduce the amount of contact between the liquid and solid. But such surfaces are delicate and prone to failure. The slippery alternative offered by LiquiGlide is a liquid-impregnated surface, shown in the bottom image. Like a superhydrophobic surface, it consists of a textured solid but one that’s filled with a liquid lubricant that preferentially wets the solid. As a result, the liquid to be shed has little to no contact with the actual solid surface and therefore slides easily off! (Image credit: LiquiGlide, source; research credit: K. Varanasi et al.; suggested by cnsidero)

How Dogs and Cats Drink

We humans do our hands-free drinking via suction, using the shape of our lips and mouths to create low pressure that draws liquids in. Dogs and cats, on the other hand, have no cheeks and, therefore, no suction. Instead, both cats (top) and dogs (bottom) drink using adhesion, or the tendency of a liquid to stick to a surface. Both species flatten part of their tongue against the water surface, then pull it up rapidly. This draws a column of water up after their tongue, which they then snap their jaws closed around. Although they use the same method, cats are daintier drinkers than dogs, which sometimes leads to the misconception that the animals drink differently. (Image credits: NYTimes, source; research credit: S. Jung et al.)

Spinning Paint
Fluid dynamical behaviors are often the result of competing forces. Here paint flung from a spinning rod illustrates the effects of adhesion, surface tension, and centrifugal force. In general, surface tension tries to hold a fluid together, and adhesion allows it to stay attached to a surface. Centrifugal force, on the other hand, tends to push the fluid outward. As the spinning rod accelerates, centrifugal force wins over adhesion and the paint spirals outward. For awhile, surface tension manages to hold the paint together, stretching it into spiraling ligaments of fluid. But when centrifugal force overpowers surface tension as well, the ligaments of paint snap into smaller droplets, still flying outward. Check out the full video for more great slow motion shots, and be sure to look at photographer Fabian Oefner’s “Black Hole“ series, which inspired the video. (Image credit: BBC Earth Unplugged, source video)

Inside a Water Blob
This new video from the Space Station shows once again that astronauts have the most fun job on–or off–the planet. In it, the Expedition 40 crew members submerge a GoPro camera in a microgravity water blob. Here on Earth, we’re used to surface tension being a minor or secondary force with most fluids we experience daily. This is because gravity often provides the overwhelming effect. But in microgravity, those effects are absent, and forces like surface tension and adhesion dominate water’s behavior. This both why the crew can make such a large water sphere hold together, and why one astronaut eventually gets his hands stuck in the sphere. (Video credit: NASA; submitted by jshoer)

Meandering Rivulet
This rivulet is the result of a horizontal liquid jet impacting a vertical pane of glass. Gravity, surface tension, adhesion, and even surface finish can affect the path the water follows. Like the meandering path of rain on a windshield, it’s hard to predict a priori where the flow will go without accounting for a myriad of seemingly inconsequential variables governing both the liquid and solid surface. (Photo credit: T. Wang)

How Patterns Repel Water
Superhydrophobic surfaces repel water. Both naturally occurring and manmade materials with this property share a common feature: micro- or nanoscale structures on their surface. Lotus and lily leaves are coated with tiny hairs, and synthetic coatings or micro-manufactured surfaces like the one in the video above can be made in the lab. This nanoscale roughness traps air between the surface and the water, preventing adhesion to the surface and enabling the water-repelling behavior we observe at the human scale. Although effective, these nanoscale structures are also extremely delicate, which makes widespread application of superhydrophobic coatings and textures difficult. (Video credit: G. Azimi et al.)

How Dogs Drink
This high-speed footage shows how a dog drinks. The dog’s tongue curls backwards, creating a large area of surface contact with the water. When the dog pulls its tongue back up, water adheres to it and is drawn upward in a column. The dog then closes its mouth around the water before it falls. Fundamentally, this is the same mechanism as the one cats use. Part of the reason that dogs are messier drinkers, though, is that the backwards curl of their tongue picks up extra water. Because the dog has no cheeks, there’s no way to move this water from the underside to the top of the tongue and so the water just falls back out. (Video credit: Oxford Scientific Films; submitted by Carolyn W.)