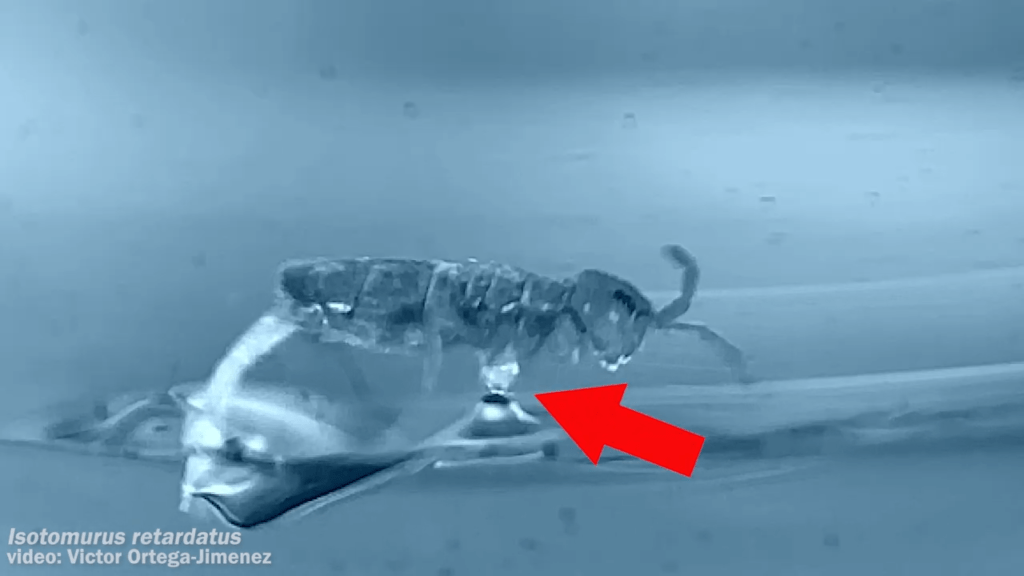
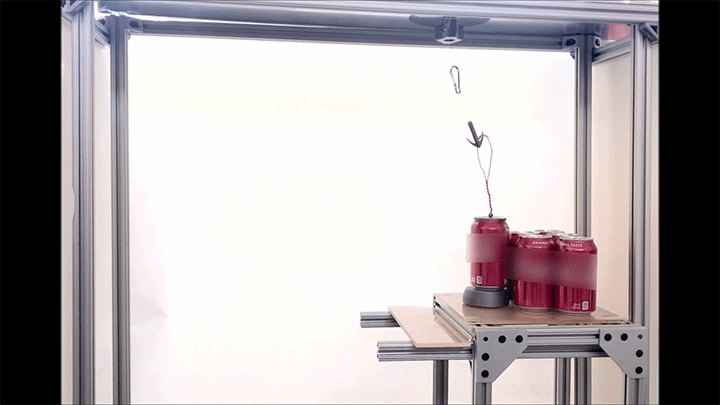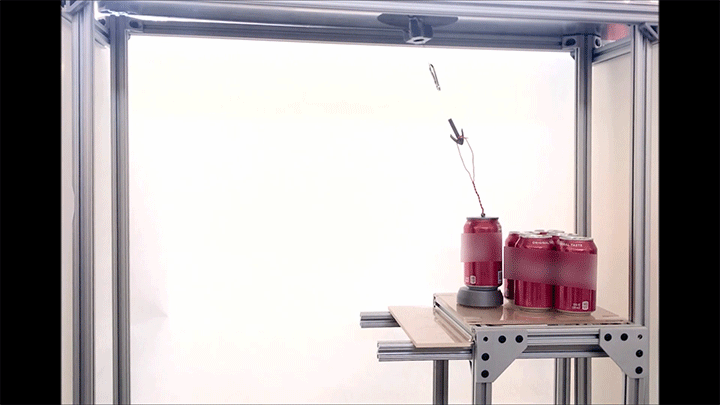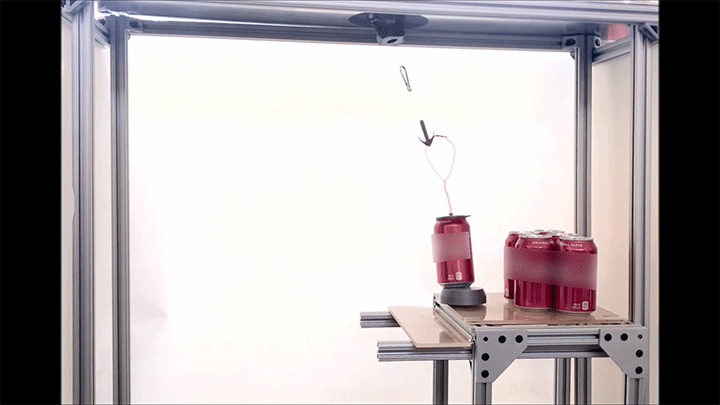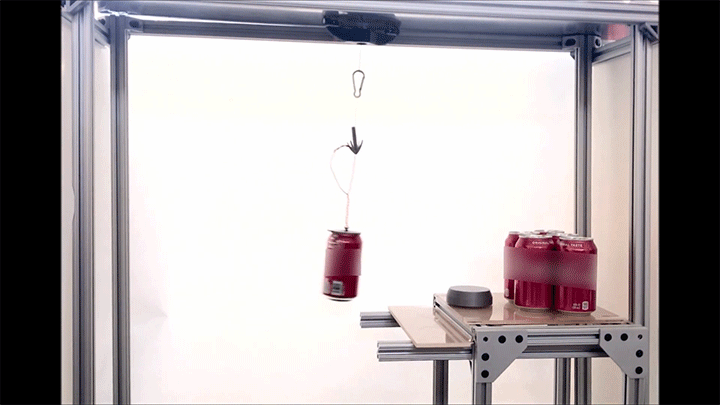
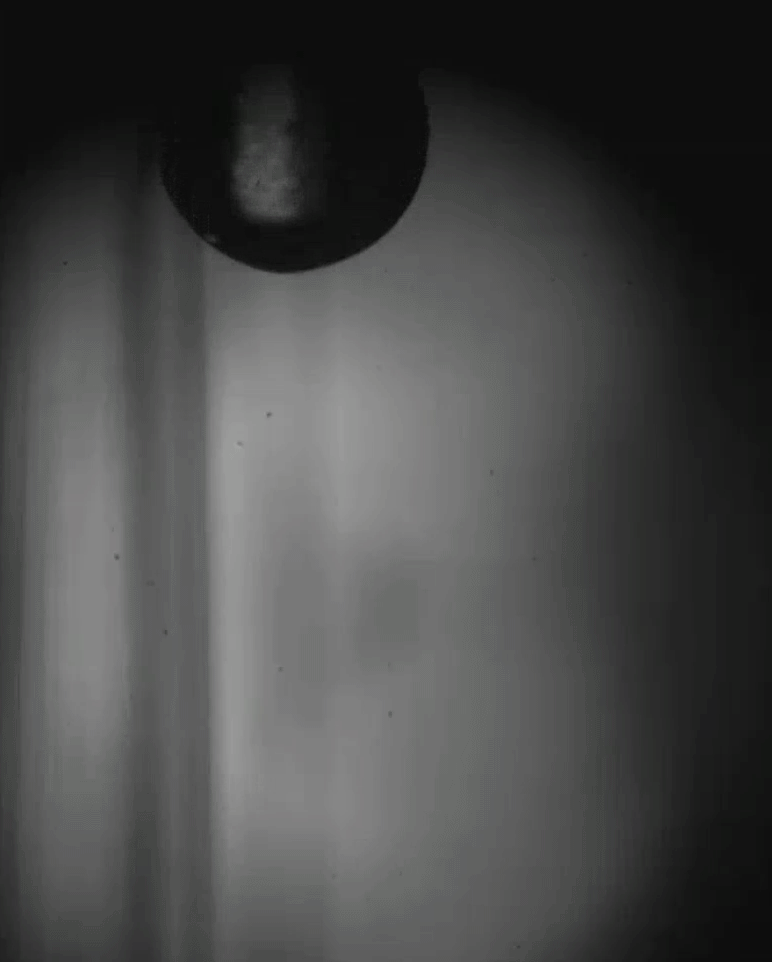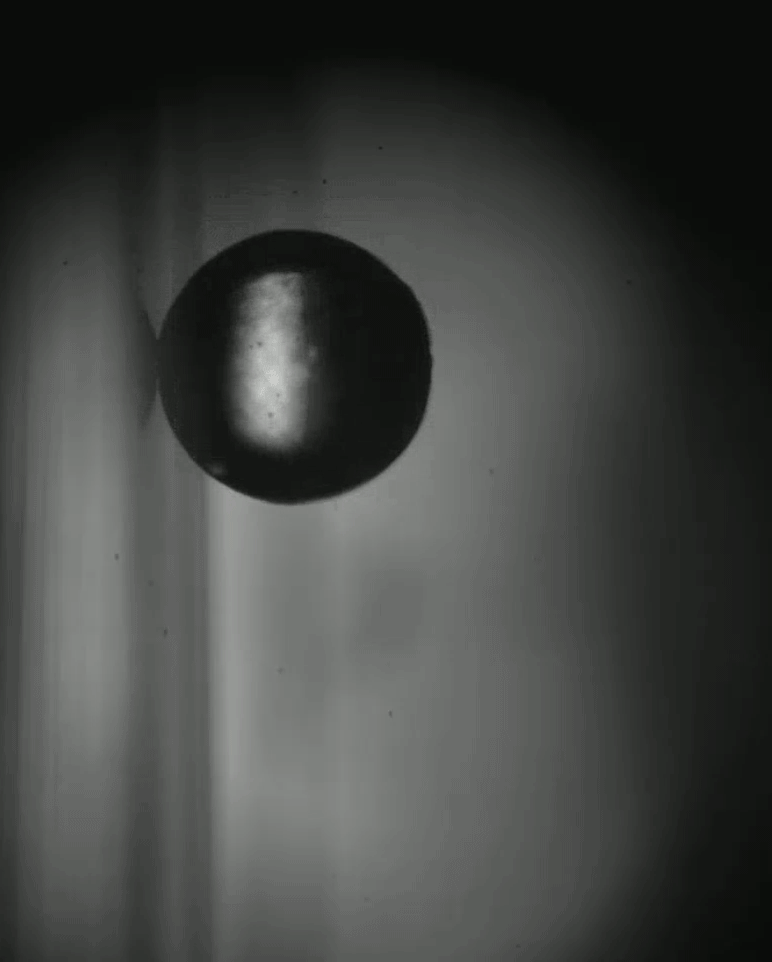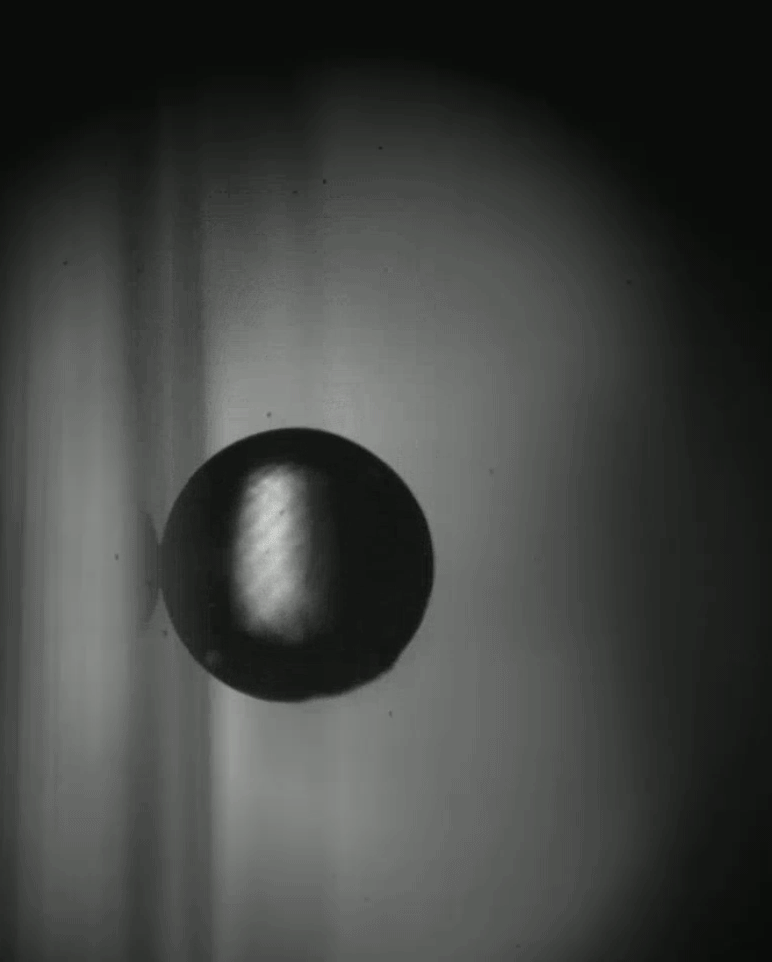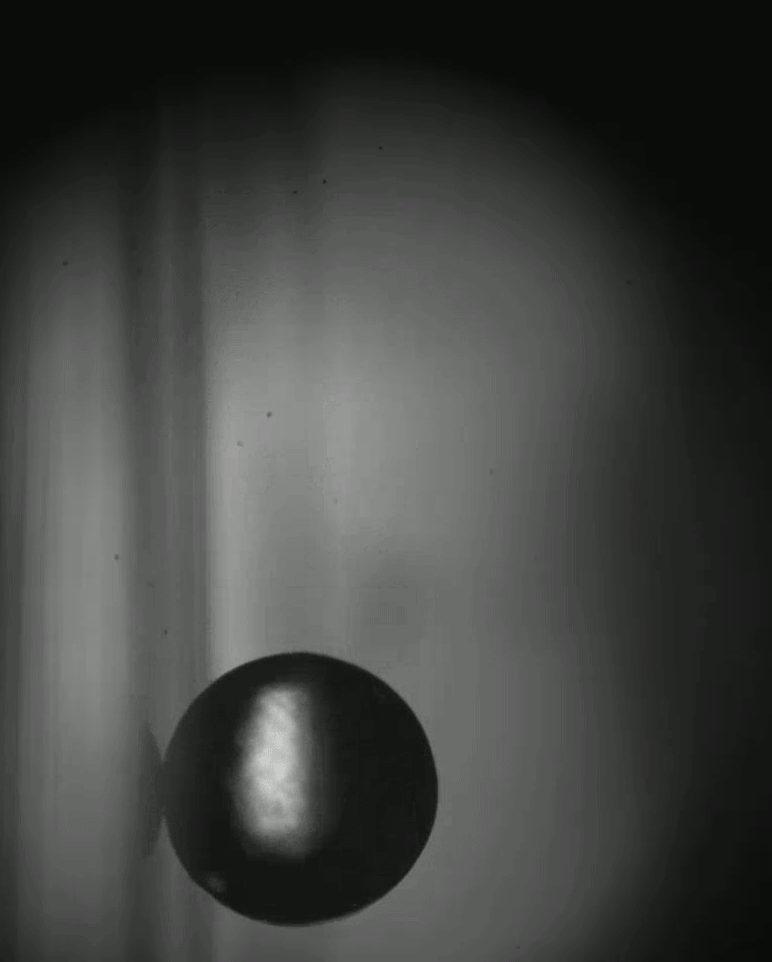Place a rigid ball on a hard vertical surface, and it will free fall. Stick a liquid drop there, and it will slide down. But researchers discovered that with a soft sphere and a soft surface, it’s possible to roll down a vertical wall. The effect requires just the right level of squishiness for both the wall and sphere, but when conditions are right, the 1-millimeter radius sphere rolls (with a little slipping) down the wall.
Rolling requires torque, something that’s usually lacking on a vertical surface. But the team found that their soft spheres got the torque needed to roll from their asymmetric contact with the surface. More of the sphere contacted above its centerline than below it. The researchers compared the way the sphere contacted the surface to a crack opening (at the back of the sphere) and a crack closing (at the front of the sphere). That asymmetry creates just enough torque to roll the sphere slowly. The team hopes their discovery opens up new possibilities for soft robots to climb and descend vertical surfaces. (Image and research credit: S. Mitra et al.; via Gizmodo)