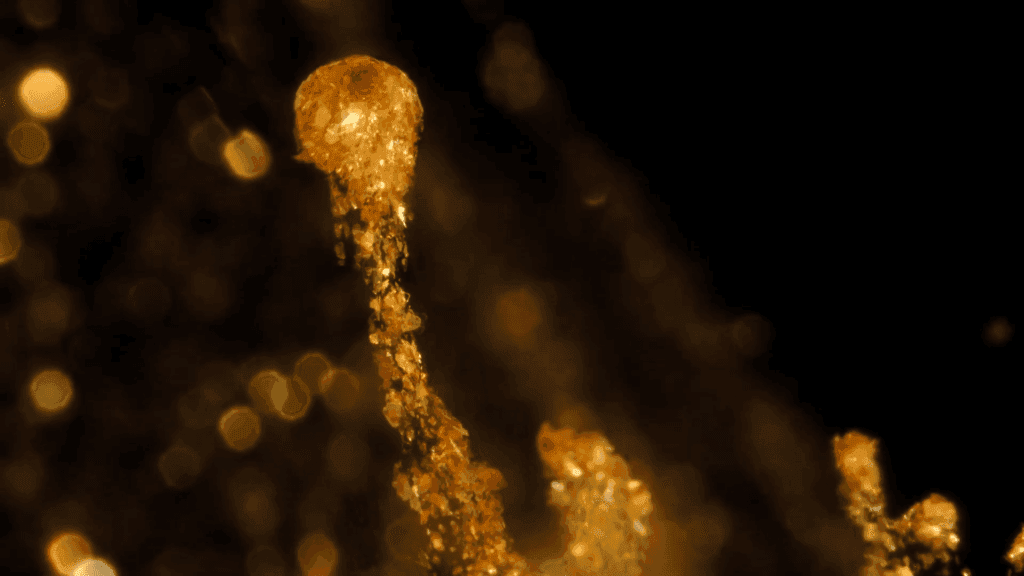Droplets of a gallium alloy are liquid at room temperature. When spiked with aluminum grains and immersed in a solution of NaOH, the droplets change shape and move in a random fashion. This video delves into the phenomenon, describing how a chemical reaction with the aluminum grains changes the local surface tension and creates Marangoni flows that make the droplets move. To get the droplet motion, you need to have the aluminum concentration just right. With too little, there’s not enough Marangoni flow. With too much, the hydrogen gas produced in the chemical reaction disrupts the droplet motion. (Video and image credit: N. Kim)
Tag: surface tension

Building a Better Fog Harp
On arid coastlines, fog rolling in can serve as an important water source. Today’s fog collectors often use tight mesh nets. The narrow holes help catch tiny water particles, but they also clog easily. A few years ago, researchers suggested an alternative design — a fog harp inspired by coastal redwoods — that used closely spaced vertical wires to capture water vapor. At small scales, this technique worked well, but once scaled up to a meter-long fog harp, the strings would stick together once wet — much the way wet hairs cling to one another.
The group has iterated on their design with a new hybrid that maintains the fog harp’s close vertical spacing but adds occasional cross-wires to stabilize. Laboratory tests are promising, with the new hybrid fog harp collecting water with 2 – 8 times the efficiency of either a conventional mesh or their original fog harp. The team notes that even higher efficiencies are possible with electrification. (Image credit: A. Parrish; research credit: J. Kaindu et al.; via Ars Technica)

How Insects Fly in the Rain
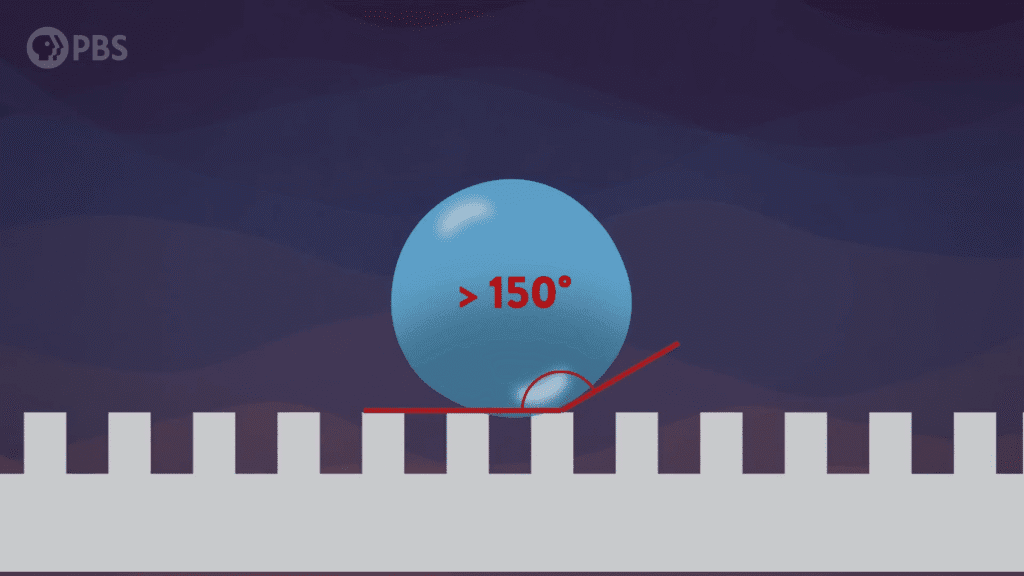
Getting caught in the rain is annoying for us but has the potential to be deadly for smaller creatures like insects. So how do they survive a deluge? First, they don’t resist a raindrop, and second, they have the kinds of surfaces water likes to roll or bounce off. The key to this second ability is micro- and nanoscale roughness. Surfaces like butterfly wings, water strider feet, and leaf surfaces contain lots of tiny gaps where air gets caught. Water’s cohesion — its attraction to itself — is large enough that water drops won’t squeeze into these tiny spaces. Instead, like the ball it resembles, a water drop slides or bounces away. (Video and image credit: Be Smart)

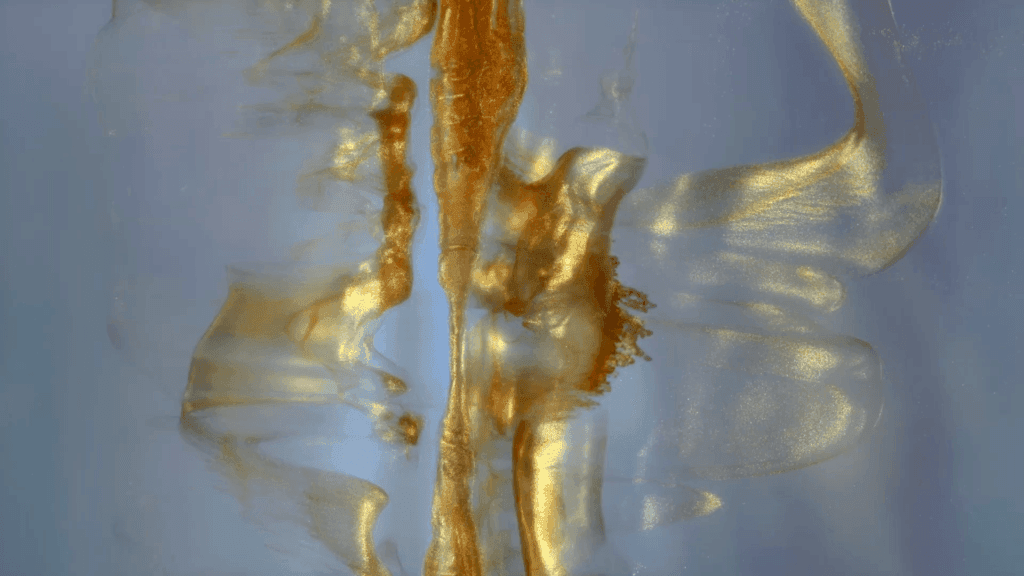
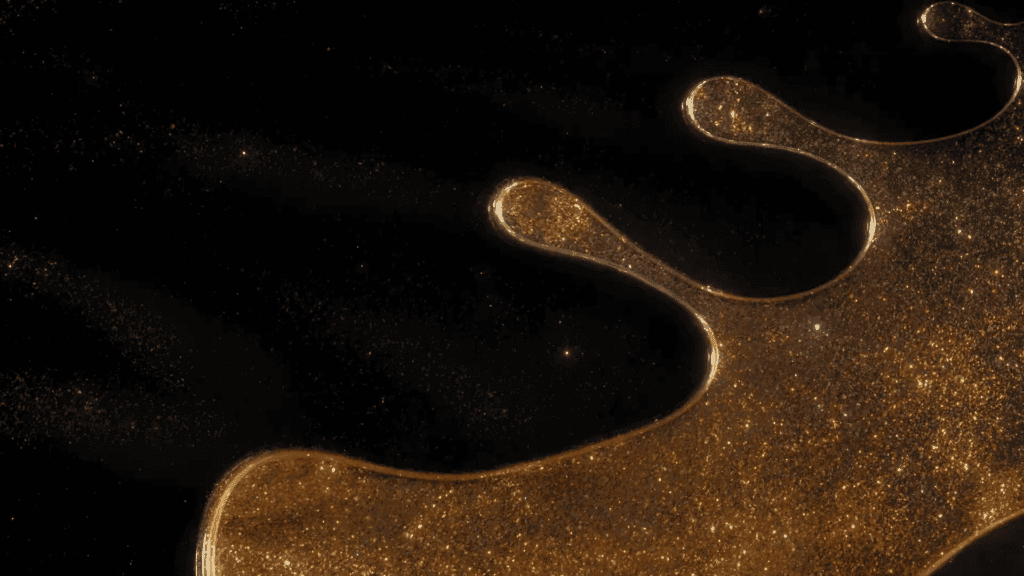
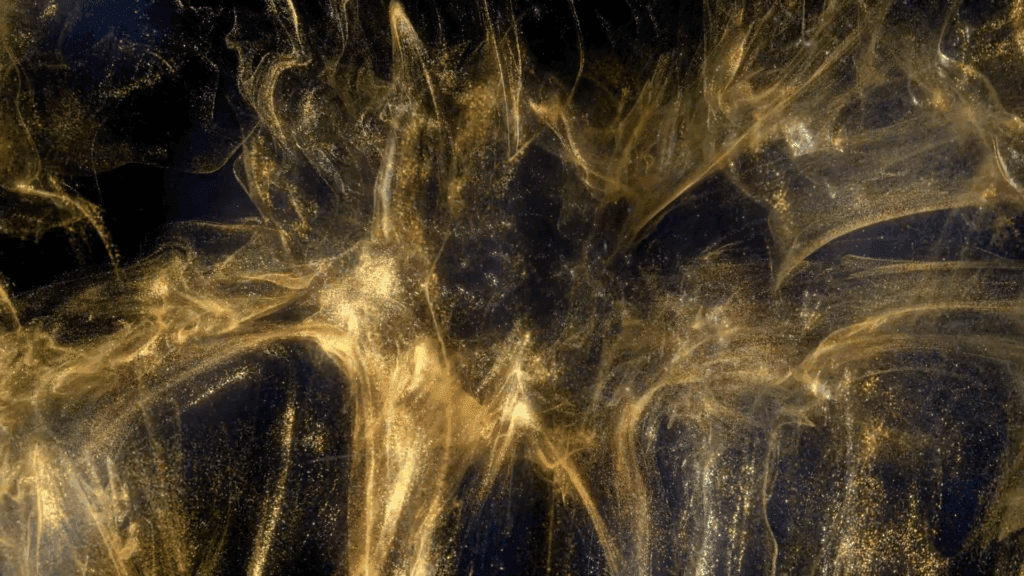
“Legend”
Filmmaker Roman De Giuli returns to his roots with this short fluid-filled film inspired by the color gold. He combines paint, ink, powders, and particles in a mix of micro- and macroscale photography. As always, the results are a mesmerizing plethora of fluid phenomena: Marangoni flows, turbulence, vorticity, viscous fingering and so much more. (Video and image credit: R. De Giuli)

Explosively Jetting
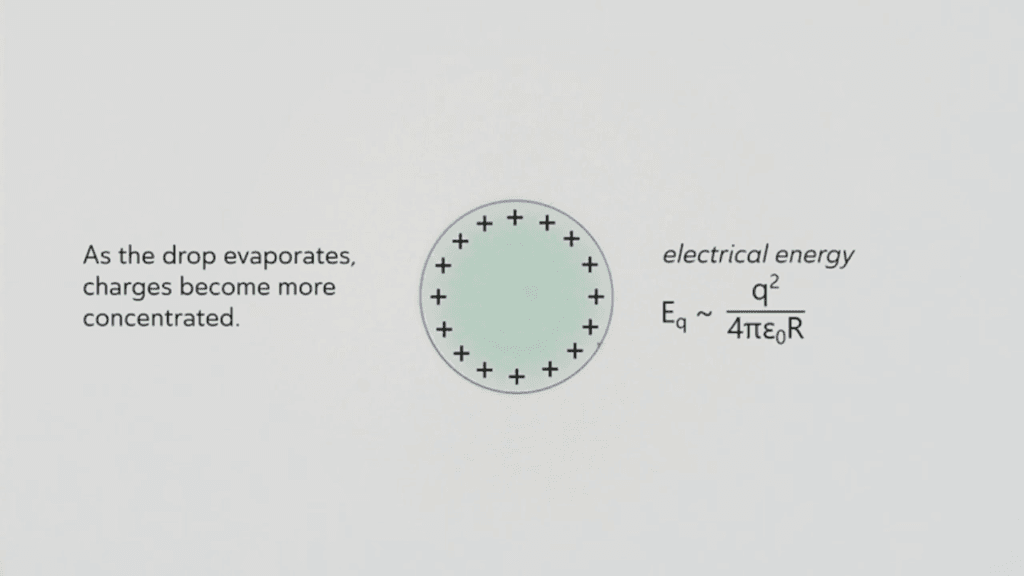
Dropping water from a plastic pipette onto a pool of oil electrically charges the drop. Then, as it evaporates, it shrinks and concentrates the charges closer and closer. Eventually, the strength of the electrical charge overcomes surface tension, making the drop form a cone-shaped edge that jets out tiny, highly-charged microdrops. Afterward, the drop returns to its spherical shape… until shrinkage builds up the charge density again. This microjetting behavior can carry on for hours! (Video and image credit: M. Lin et al.; research preprint: M. Lin et al.)

Flow Behind Viscous Fingers
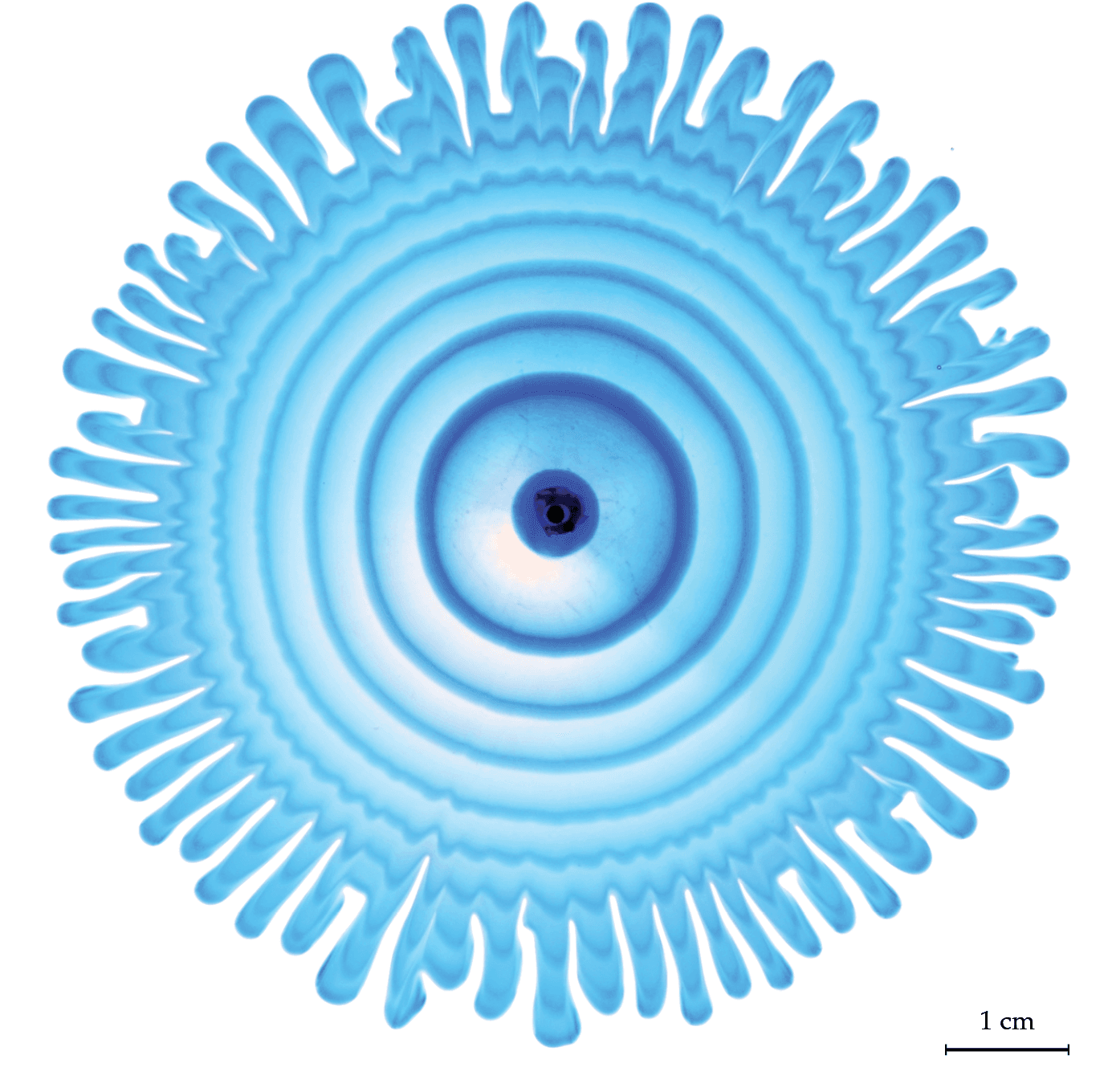
Nature is full of branching patterns: trees, lighting, rivers, and more. In fluid dynamics, our prototypical branching pattern is the Saffman-Taylor instability, created when a less viscous fluid is injected into a more viscous one in an confined space. Most attention in this problem has gone to the branching interface where the two fluids meet, but recently, a team has examined the flow away from the fingers by alternately injecting dyed and undyed fluid to visualize what goes on. That’s what we see here. Notice how the central dye rings, far from the branching fingers, still appear circular. Yet, even a few centimeters away from the fingers, the dye is starting to show ripples that correspond to the fingers. That’s an indication that the pressure gradient generated at the tips of the fingers is pretty far-reaching! (Image and research credit: S. Gowen et al.)

The Mystery of the Binary Droplet
What goes on inside an evaporating droplet made up of more than one fluid? This is a perennially fascinating question with lots of permutations. In this one, researchers observed water-poor spots forming around the edges of an evaporating drop, almost as if the two chemicals within the drop are physically separating from one another (scientifically speaking, “undergoing phase separation“). To find out if this was really the case, they put particles into the drop and observed their behavior as the drop evaporated. What they found is that this is a flow behavior, not a phase one. The high concentration of hexanediol near the edge of the drop changes the value of surface tension between the center and edge of the drop. And that change is non-monotonic, meaning that there’s a minimum in the surface tension partway along the drop’s radius. That surface tension minimum is what creates the separated regions of flow. (Video and image credit: P. Dekker et al.; research pre-print: C. Diddens et al.)

Within a Drop
In this macro video, various chemical reactions swirl inside a single dangling droplet. Despite its tiny size, quite a lot can go on in a drop like this. Both the injection of chemicals and the chemical reactions themselves can cause the flows we see here. Surface tension variations and capillary waves on the exterior of the drop can play a role, too. Just because a flow is tiny doesn’t mean it’s simple. (Video and image credit: B. Pleyer; via Nikon Small World in Motion)

Chemical reactions swirl within a single, hanging droplet. 
Active Cheerios Self-Propel
The interface where air and water meet is a special world of surface-tension-mediated interactions. Cereal lovers are well-aware of the Cheerios effect, where lightweight O’s tend to attract one another, courtesy of their matching menisci. And those who have played with soap boats know that a gradient in surface tension causes flow. Today’s pre-print study combines these two effects to create self-propelling particle assemblies.
The team 3D-printed particles that are a couple centimeters across and resemble a cone stuck atop a hockey puck. The lower disk area is hollow, trapping air to make the particle buoyant. The cone serves as a fuel tank, which the researchers filled with ethanol (and, in some cases, some fluorescent dye to visualize the flow). Like soap, ethanol’s lower surface tension disrupts the water’s interface and triggers a flow that pulls the particle toward areas with higher surface tension. But, unlike soap, ethanol evaporates, effectively restoring the interface’s higher surface tension over time.
With multiple self-propelling particles on the interface, the researchers observed a rich series of interactions. Without their fuel, the Cheerios effect attracted particles to each other. But with ethanol slowly leaking out their sides, the particles repelled each other. As the ethanol ran out and evaporated, the particles would again attract. By tweaking the number and position of fuel outlets on a particle, the researchers found they could tune the particles’ attractions and motility. In addition to helping robots move and organize, their findings also make for a fun educational project. There’s a lot of room for students to play with different 3D-printed designs and fuel concentrations to make their own self-propelled particles. (Research and image credit: J. Wilt et al.; via Ars Technica)

Predicting Droplet Sizes
Squeeze a bottle of cleaning spray, and the nozzle transforms a liquid jet into a spray of droplets. These droplets come in many sizes, and predicting them is difficult because the droplets’ size distribution depends on the details of how their parent liquid broke up. Shown above is a simplified experimental version of this, beginning with a jet of air striking a spherical water droplet on the far left. In less than 3 milliseconds, the droplet has flattened into a pancake shape. In another 4 milliseconds, the pancake has ballooned into a shape called a bag, made up of a thin, curved water sheet surrounded by a thicker rim. A mere 10 milliseconds after the jet and drop first meet, the liquid is now a spray of smaller droplets.
Researchers have found that the sizes of these final droplets depend on the balance between the airflow and the drop’s surface tension; these two factors determine how the drop breaks up, whether that’s rim first, bag first, or due to a collision between the bag and rim. (Image credit: I. Jackiw et al.; via APS Physics)