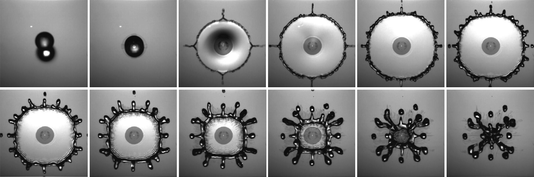
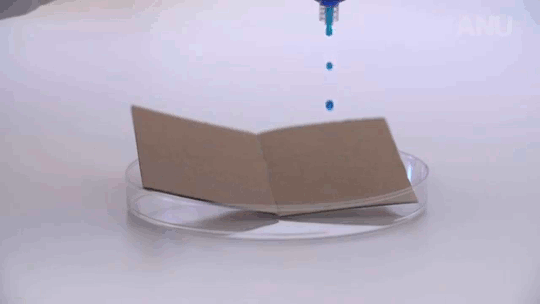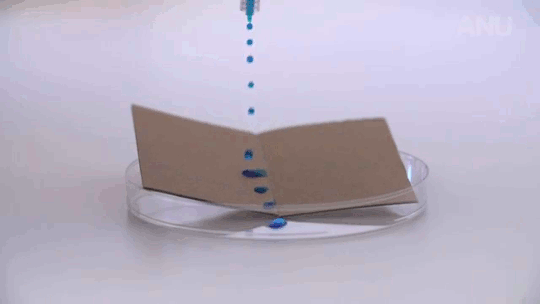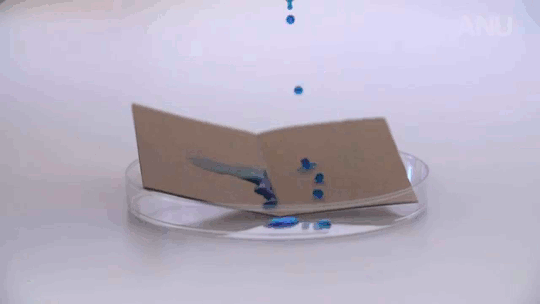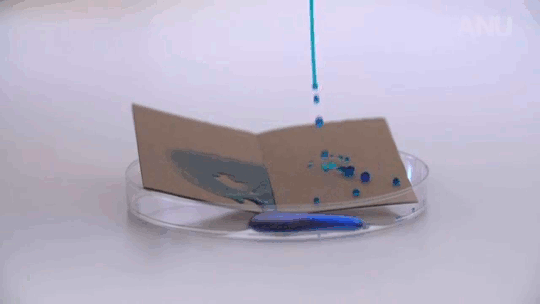
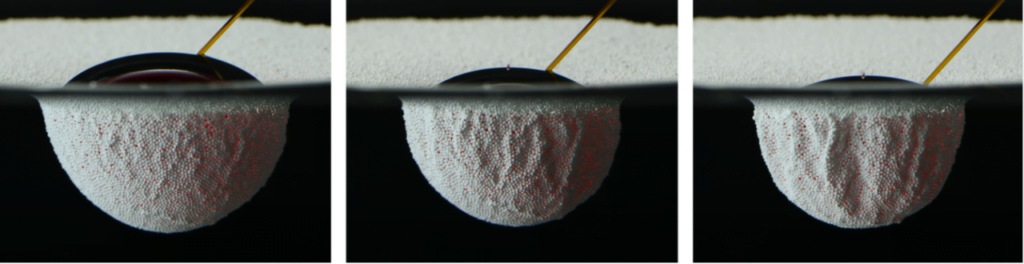
Many insects are known to quest underwater, but few are as adept at it as the alkali fly. This species has taken common attributes among flies – being covered in tiny hairs and a waxy layer – and really upped the ante. Their extra hairiness and extra waxiness make them extremely difficult to get wet, even in the excessively salty and alkaline waters of California’s Mono Lake, which are enough to defeat all but algae, brine shrimp, bacteria, and alkali flies.
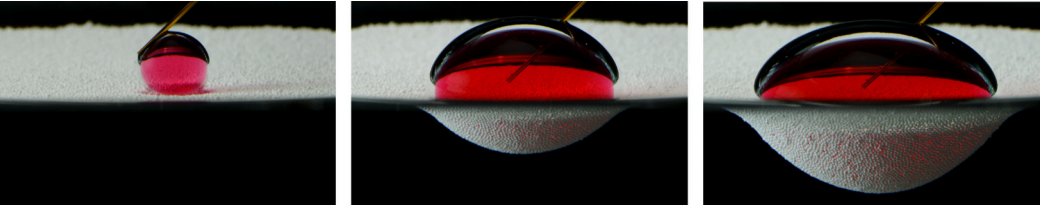
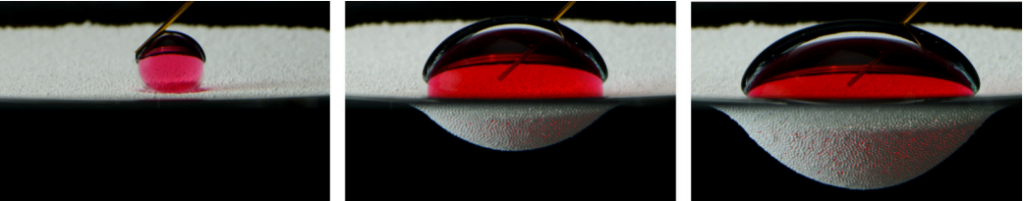
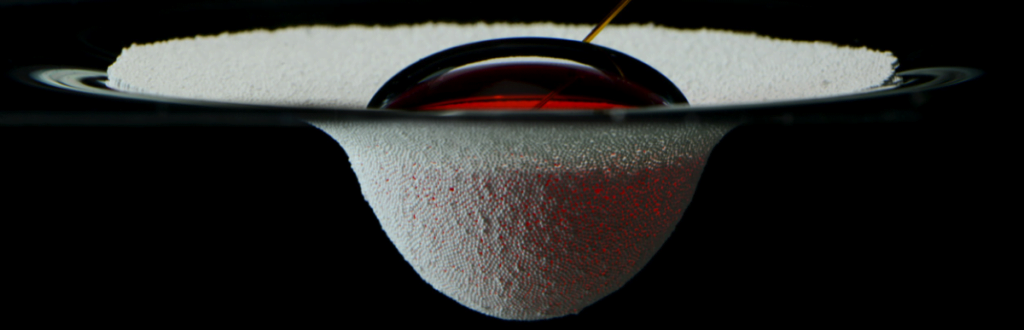
Staying dry is a challenge, but only one of many this insect tackles. The combination of hair and wax over the insect makes it superhydrophobic, coating it in a silvery layer of air as it crawls below the surface. All that air is buoyant, so to walk underwater, the fly has to exert forces up to 18 times its body weight just to keep from popping back up to the surface.
The shimmering bubble also helps the fly breathe. Insect respiratory systems use openings all over the exoskeleton to exchange oxygen with the ambient atmosphere via diffusion. While diffusion of oxygen does still happen underwater, it’s a much slower process there. The air sheath around the fly creates a large surface area for oxygen to diffuse, which helps counter the lower diffusion rate. Inside the sheath, the fly breathes as it normally does. (Image and research credit: F. van Breugel and M. Dickinson; via Gizmodo; submitted by @1307phaezr)