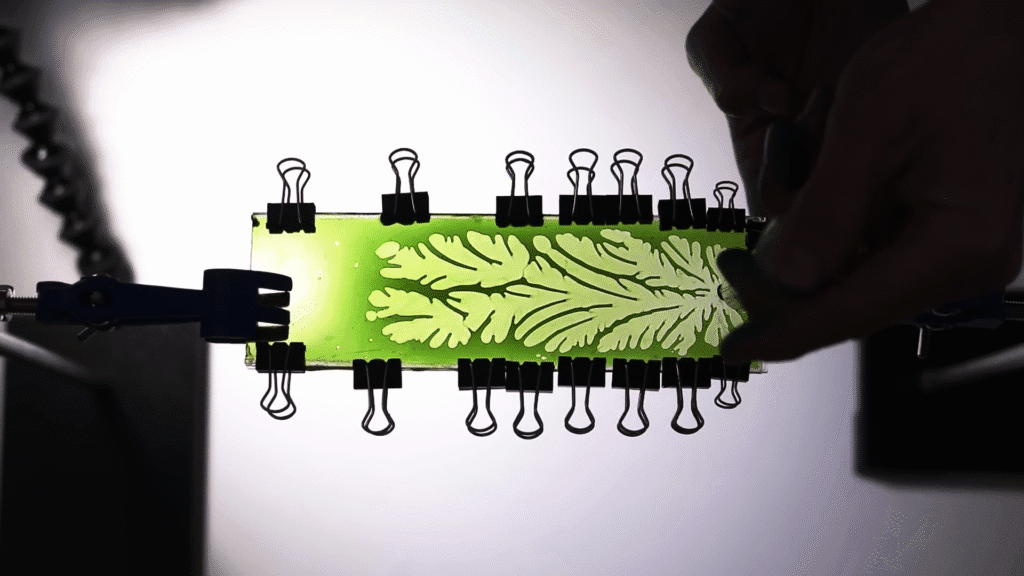
As bizarre as the branching fractal fingers of the Saffman-Taylor instability look, they’re quite a common phenomenon. In his video, Steve Mould demonstrates how to make them by sandwiching a viscous liquid like school glue between two acrylic sheets and then pulling them apart. The more formal lab-version of this is the Hele-Shaw cell, which he also demonstrates. But you may have come across the effect when pealing up a screen protector or in dealing with a cracked phone screen. In all of these cases, a less viscous fluid — specifically air — is forcing its way into a more viscous fluid, something that it cannot manage without the fluid interface fracturing. (Video and image credit: S. Mould)
Tag: Saffman-Taylor instability

Flow Behind Viscous Fingers
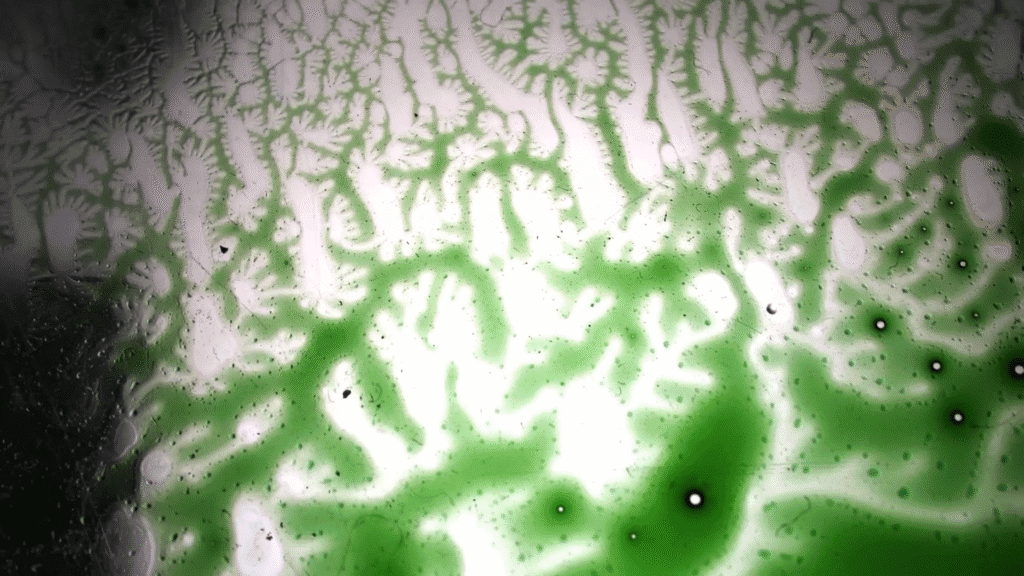
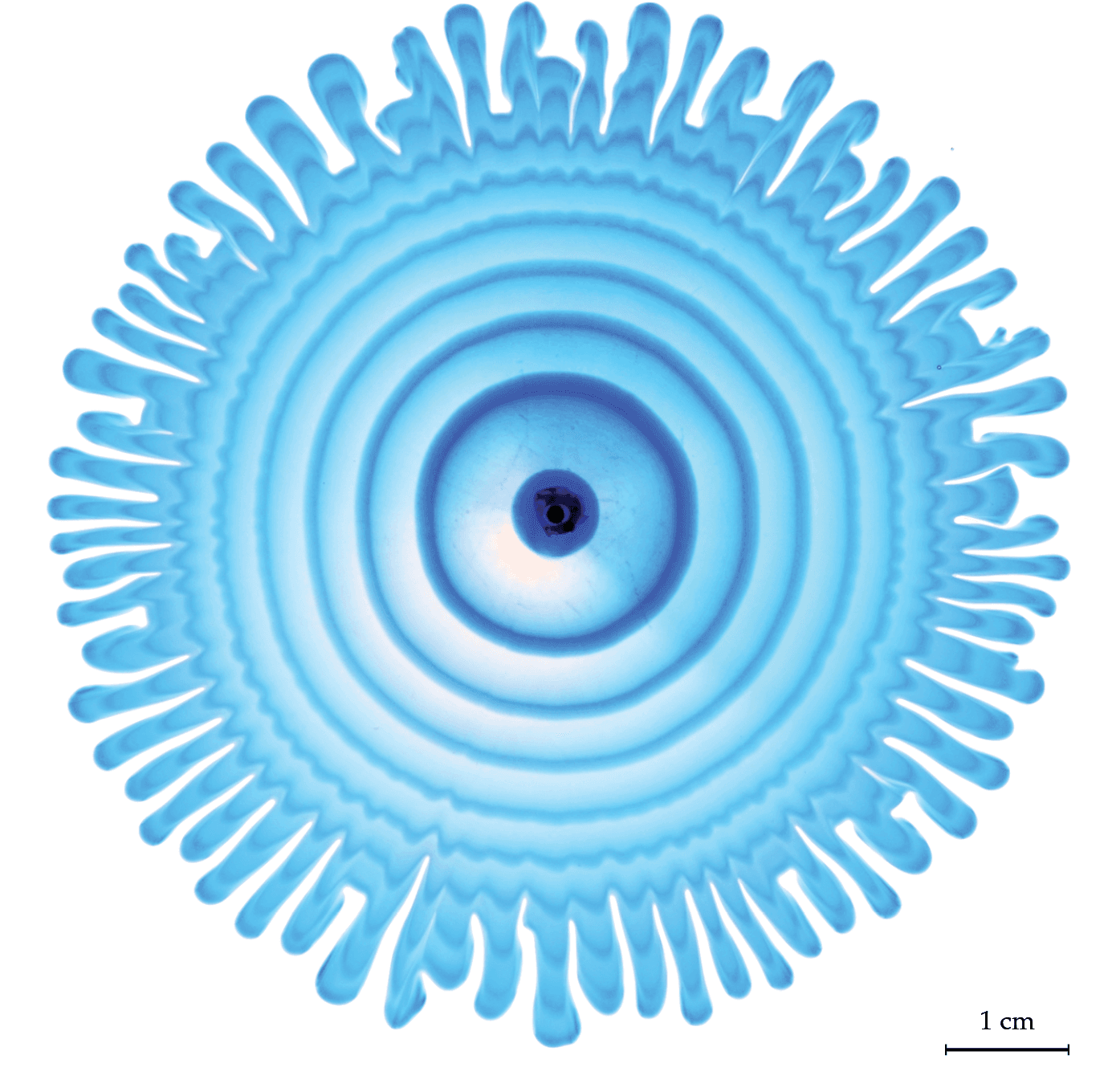
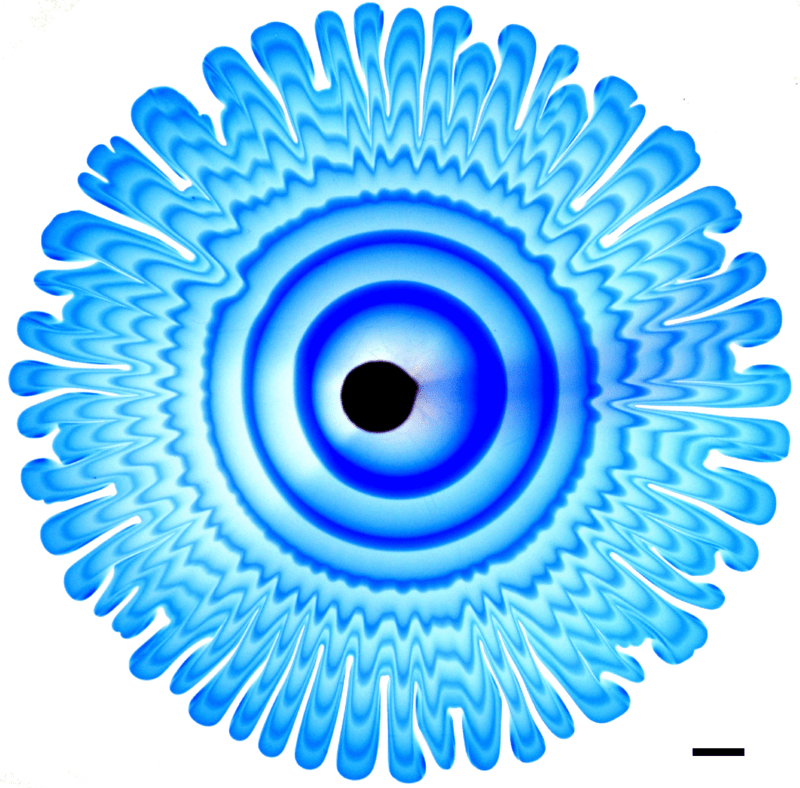
Nature is full of branching patterns: trees, lighting, rivers, and more. In fluid dynamics, our prototypical branching pattern is the Saffman-Taylor instability, created when a less viscous fluid is injected into a more viscous one in an confined space. Most attention in this problem has gone to the branching interface where the two fluids meet, but recently, a team has examined the flow away from the fingers by alternately injecting dyed and undyed fluid to visualize what goes on. That’s what we see here. Notice how the central dye rings, far from the branching fingers, still appear circular. Yet, even a few centimeters away from the fingers, the dye is starting to show ripples that correspond to the fingers. That’s an indication that the pressure gradient generated at the tips of the fingers is pretty far-reaching! (Image and research credit: S. Gowen et al.)

Peering Inside Viscous Fingering
Viscous fingers form when a low-viscosity fluid is pumped into a narrow, viscous-fluid-filled gap. The branching pattern that forms depends on the ratio of the two viscosities, among other factors. To better understand what goes on inside these fingers, researchers carefully alternated injecting dyed and undyed fluid. This creates a pattern of concentric rings that deform as the fingers spread.
In this particular study, the initial fluid and injected fluids are miscible, meaning that they can mix into one another. In modeling their experiments, the team found that this mixing created stratification — i.e., layers of fluids with different densities — in the narrow gap between their plates. The stratification’s effects were large enough that the model required a correction term for them; that’s a bit surprising because we’d usually expect that the tiny third-dimension of the gap would be too small to matter! (Image and research credit: S. Gowan et al.)

Viscous Fireworks
Inject a less viscous fluid into a gap filled with a more viscous fluid, and you’ll get finger-like patterns spreading radially. Here, researchers put a twist on this viscous fingering by taking turns injecting different liquids. Each injection cycle disrupts what came before, layering fingering patterns on fingering patterns. The results resemble fireworks. Happy 4th of July! (Image credit: C. Chou et al.)

Evolving Fingers
If you sandwich a viscous fluid between two plates and inject a less viscous fluid, you’ll get viscous fingers that spread and split as they grow. This research poster depicts that situation with a slight twist: the viscous fluid (transparent in the image) is shear-thinning. That means its viscosity drops when it’s deformed. In this situation, the fingers formed by the injected (blue) fluid start out the way we’d expect: splitting as they grow (inner portion of the composite image). But then, the tip-splitting stops and the fingers instead elongate into spikes (middle ring). Eventually, as the outer fluid’s viscosity drops further, the fingers round out and spread without splitting (outer arc of the image). (Image credit: E. Dakov et al.; via GoSM)

Controlling Finger Formation
When gas is injected into thin, liquid-filled gaps, the liquid-gas interface can destabilize, forming distinctive finger-like shapes. In laboratories, this mechanism is typically investigated in the gap between two transparent plates, a setup known as a Hele-Shaw cell. In the past, researchers looking to control the instability have explored how surface tension, viscosity, and the elasticity of the gap itself affect the flows. But a new set of studies look at the compressibility of the gas being injected.
The team found that viscous fingers formed later the higher the gas’s compressibility. That provides a potential control knob for people trying to exploit the mechanism, especially geologists. For geologists trying to extract oil, viscous fingering is detrimental, but, on the flip side, viscous fingers are desirable when injecting carbon dioxide for sequestration. With these results, users can tweak their injection characteristics to match their goals. (Image credit: C. Cuttle et al.; research credit: C. Cuttle et al. and L. Morrow et al.; via APS Physics)

Instabilities on Instabilities
The world of fluid instabilities is a rich one. Combine fluids with differing viscosities, densities, or flow speeds and they’ll often break down in picturesque and predictable manners. Here, researchers explore the Rayleigh-Taylor instability (RTI), which occurs when a denser fluid sits above a less dense one (in a gravitational field). It’s an extremely common instability, showing up in both the cream in your ice coffee and the shape of a supernova’s explosion. It’s very difficult to set up and observe, though, which is where the real cleverness of this experiment stands out.
To study the RTI, these researchers first created another instability, the Saffman-Taylor instability. They filled the space between two glass plates with a viscous fluid, then injected a less viscous one. That created the distinctive viscous fingering pattern seen in the top image. In addition to being less viscous, the injected fluid was also less dense. As it pushed into the original fluid, it displaced some of it, creating a three-layer structure with dense fluid over less-dense fluid over dense fluid. That laid the groundwork for the Rayleigh-Taylor instability form.

A side-view through the fluid mixture shows the characteristic mushroom-like plume of the Rayleigh-Taylor instability. Check out the cell-like pattern distributed across the fluid in the top image. These are plumes formed in the RTI as dense fluid sinks into the less-dense fluid below it. From the side (see second image), each plume takes on the distinctive mushroom-like shape of a Rayleigh-Taylor instability. Given time, the two fluids mix and the cellular pattern disappears. But until then, this set-up uses one instability to study a second one. How cool is that?! (Image and research credit: S. Alqatari et al., see also)

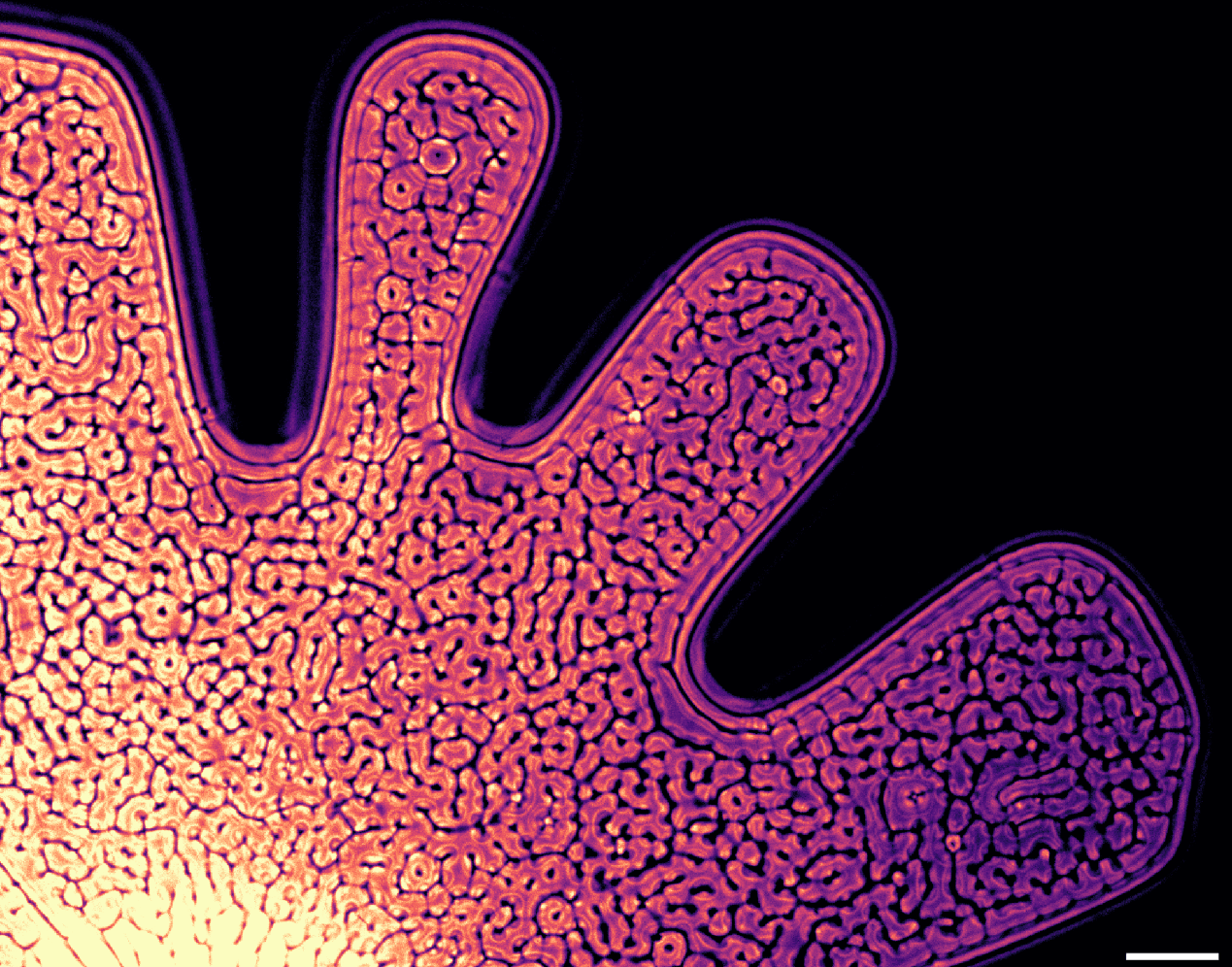
Saffman-Taylor Instability
Air and blue-dyed glycerin squeezed between two glass plates form curvy, finger-like protrusions. This is a close-up of the Saffman-Taylor instability, a pattern created when a less viscous fluid — here, air — is injected into a more viscous one. If you reverse the situation and inject glycerin into air, you’ll get no viscous fingers, just a stable, expanding circle. Although you sometimes come across this instability in daily life — like in a cracked smartphone screen — the major motivation for studying this phenomenon historically has been oil and gas extraction. (Image credit: T. Pohlman et al.)

Inside Viscous Fingers
Sandwich a viscous fluid between two transparent plates and then inject a second, less viscous fluid. This is the classic set-up for the Saffman-Taylor instability, a well-studied flow in which the interface between the two fluids forms a wavy edge that develops into fingers. Despite its long history, though, there is still more to learn, as shown in this video. Here, researchers alternately injected a dyed and undyed version of the less viscous fluid. The result (Image 3) is a set of concentric dye rings that show how the fluid moves far from the fingers along the edge. Notice that the waviness of the fingers appears in the flowing fluid well before it approaches the interface. (Image and video credit: S. Gowan et al.)

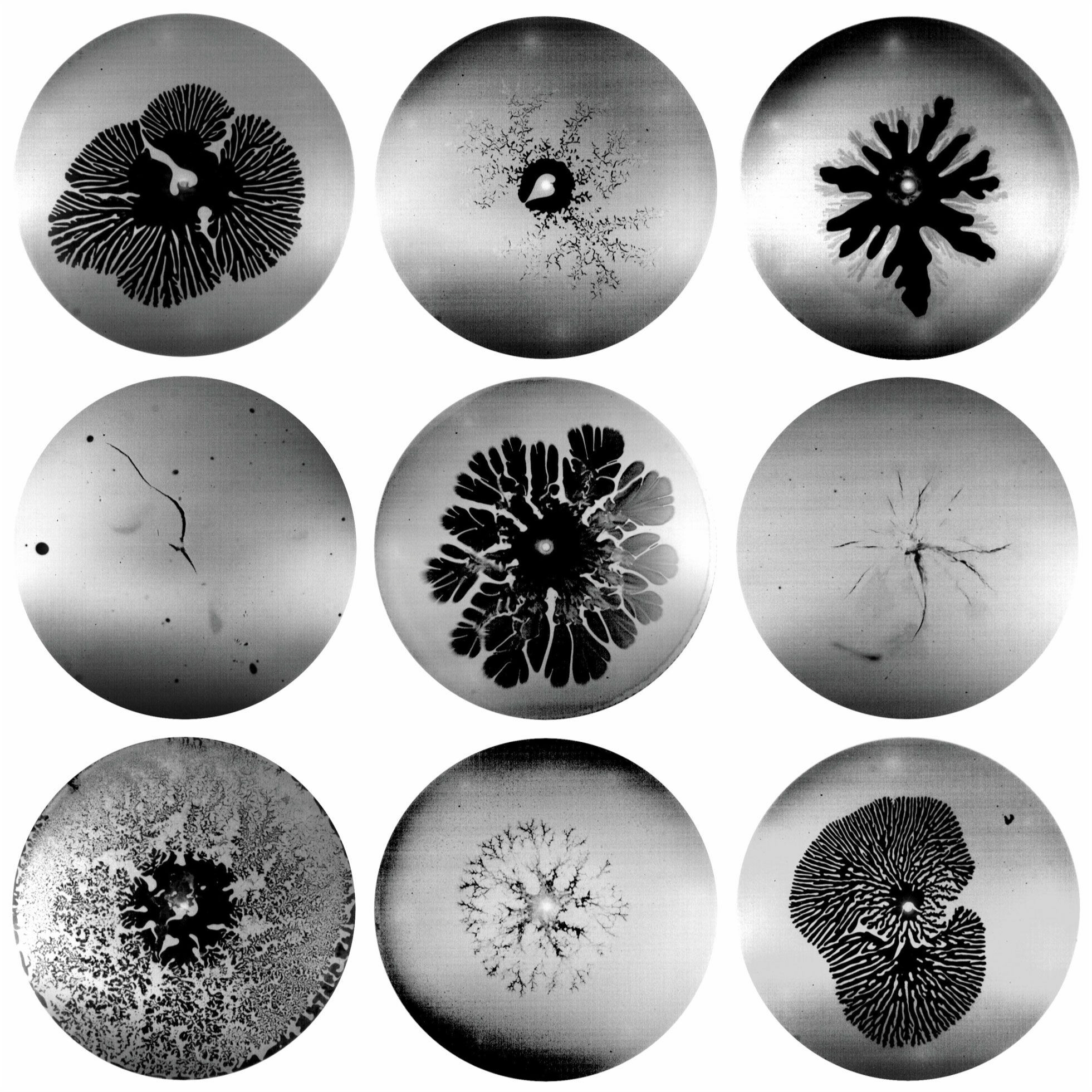
Solid, Liquid, Both?
Materials like oobleck — a suspension of cornstarch particles in water — are tough to classify. In some circumstances, they behave like a fluid, but in others, they act like a solid. Here researchers sandwiched a thin layer of oobleck between glass plates and injected air into the mixture. For a fluid, this setup creates a classic Saffman-Taylor instability where rounded fingers of air push their way into the more viscous fluid. And, indeed, for low air pressures and low concentrations of cornstarch, the oobleck forms these viscous fingers. You can see examples in the top row’s first and third image, the second row’s middle image, and the bottom row’s third image.
Injecting air at high pressures and high cornstarch concentrations fractures the oobleck like a solid (middle row, first and third images). At intermediate pressures and concentrations, the oobleck forms a pattern called dendritic fracturing, where new branches can grow perpendicularly to their parent branch. Examples of this pattern are in the top row’s second image and the bottom row’s first and second images. (Image and research credit: D. Ozturk et al.; via Physics Today)