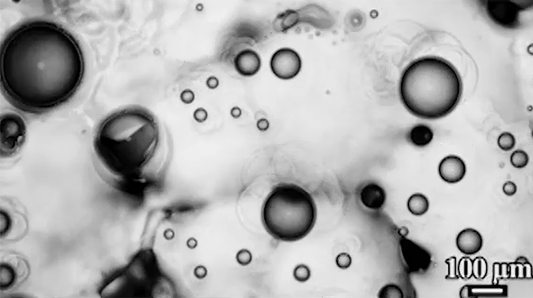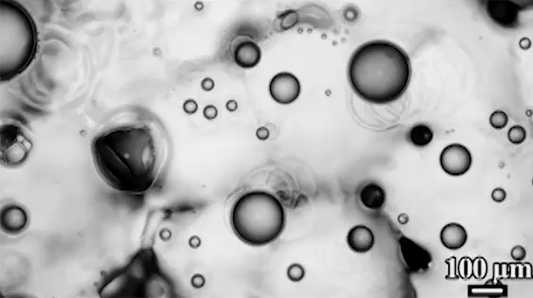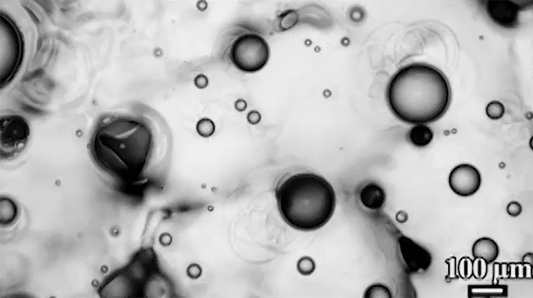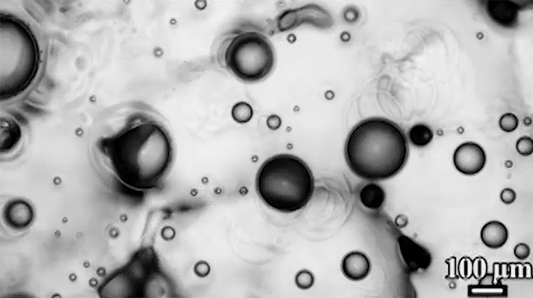
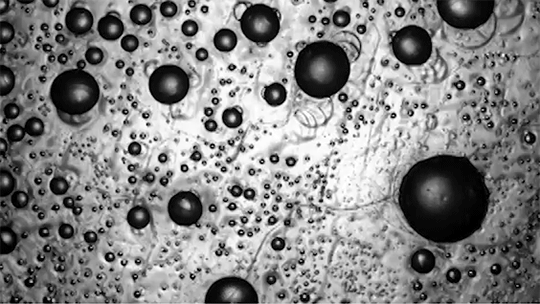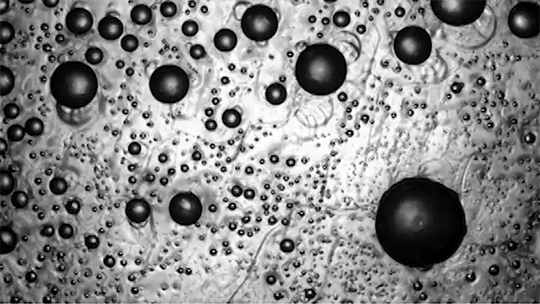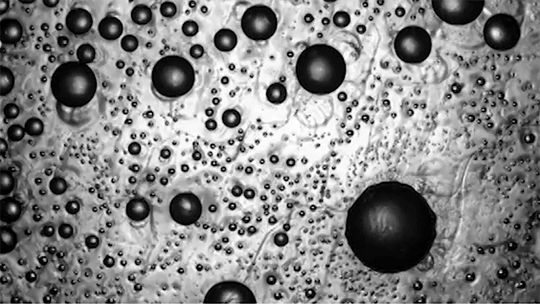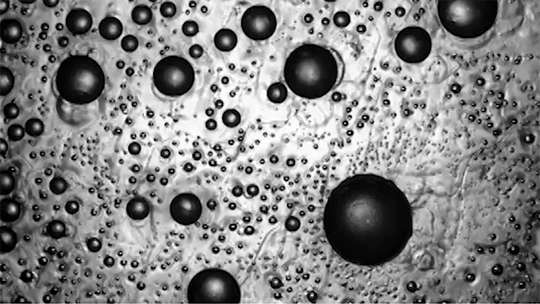
Many droplets can self-propel, often through the Leidenfrost effect and evaporation. But now researchers have observed freezing droplets that self-propel, too. The discovery came when observing the freezing of supercooled water drops inside a vacuum chamber. The researchers kept losing track of drops that seemingly disappeared. Upon closer inspection, though, they found that the drops weren’t shattering; they were flying away as they froze.
Inside a drop, freezing starts at a point, the nucleation point, and spreads from there. But the nucleation point isn’t always at the center of the drop. This asymmetry, the researchers found, is at the heart of the drop’s propulsion. When ice nucleates, the phase change releases heat that increases the drop’s evaporation rate, which can impart momentum to the drop. For an off-center nucleation, that momentum is enough to send the drop shooting off at nearly 1 meter per second. (Image credit: SpaceX; research credit: C. Stan et al.; via APS Physics)