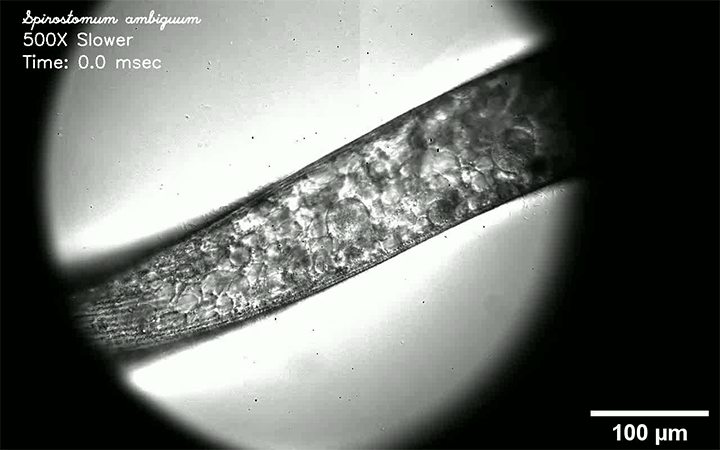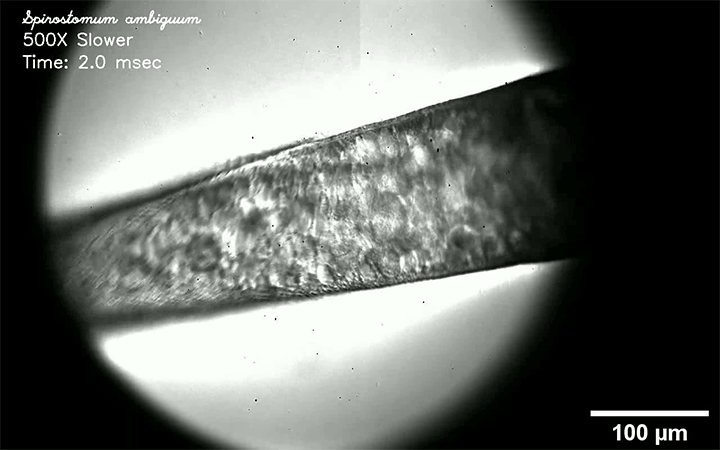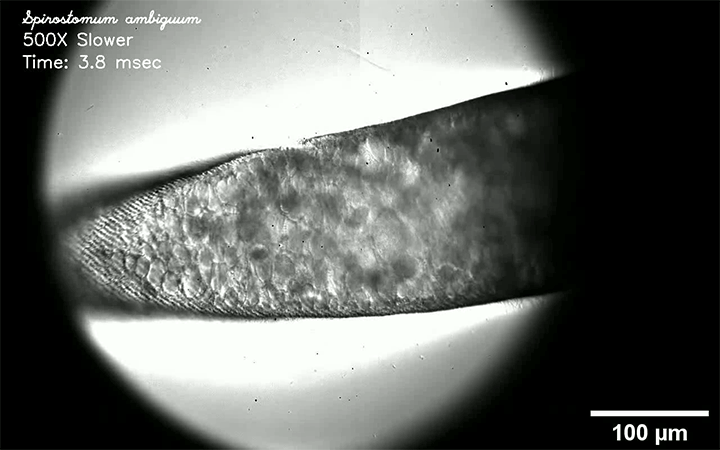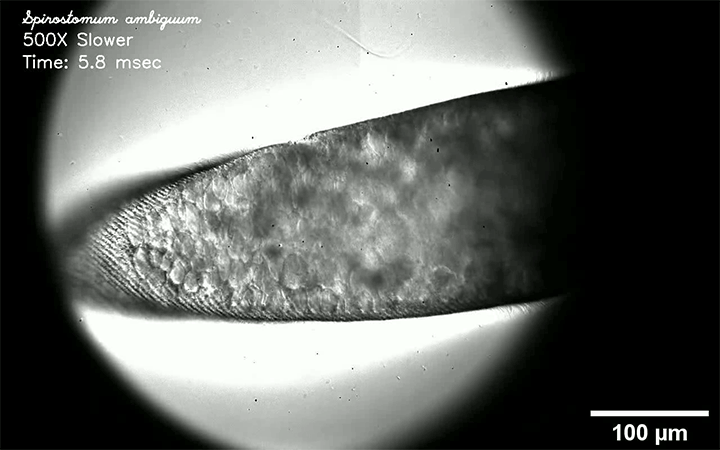
Fluids like air and water are called Newtonian because their viscosity does not vary with the force that’s applied to them. But many common fluids — almost everything in your fridge or bathroom drawer, for example — are non-Newtonian, meaning that their viscosity changes depending on how they’re deformed.
Non-Newtonian droplets can behave very differently than Newtonian ones, as this video demonstrates. Here, their fluid of choice is water with varying amounts of silica particles added. Depending on how many silica particles are in the water, the behavior of an impacting drop varies from liquid-like to completely solid and everything in between. Why such a great variation? It all has to do with how quickly the droplet tries to deform and whether the particles within it can move in that amount of time. Whenever they can’t, they jam together and behave like a solid. (Image, video, and research credit: S. Arora and M. Driscoll)