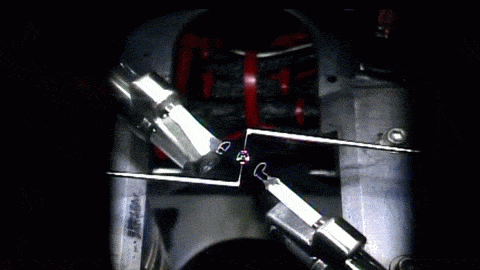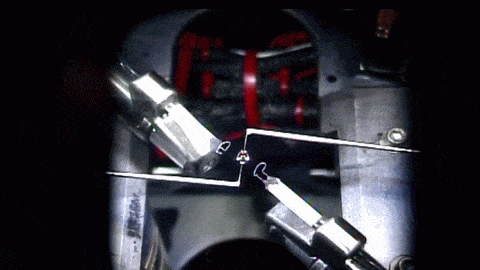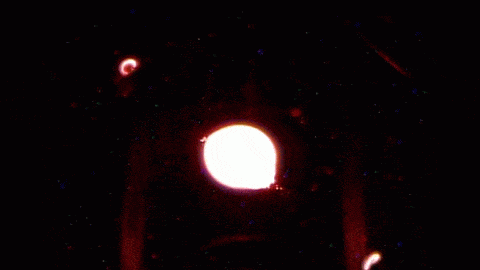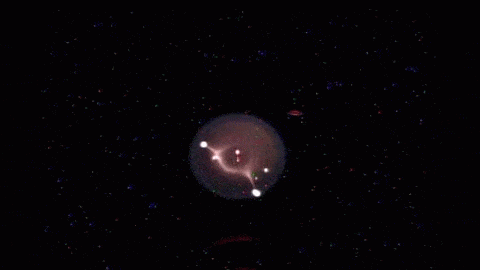
How a simple drop of water sits on a surface is a strangely complicated question. The answer depends on the droplet’s size, its chemistry, the roughness of the surface, and what kind of material it’s sitting on. Vetting the mathematical models that describe these behaviors is especially difficult since droplets often get stuck, or “pinned,” along their contact line where water, air, and surface meet.
To get around this issue, researchers sent their experiment to the International Space Station, asking astronauts to run the tests for them. Without gravity‘s influence squishing drops, the astronauts could use much larger droplets than they could on Earth. Larger drops are less likely to get pinned by a stray surface defect, so on the space station, astronauts could place droplets on a vibrating platform and observe their contact line freely moving as the drop changed shape. Under these conditions, the experiment tested many surfaces with different wetting characteristics, thereby gathering data to test models we cannot easily confirm on Earth. (Image and research credit: J. McCraney et al.; via APS Physics)