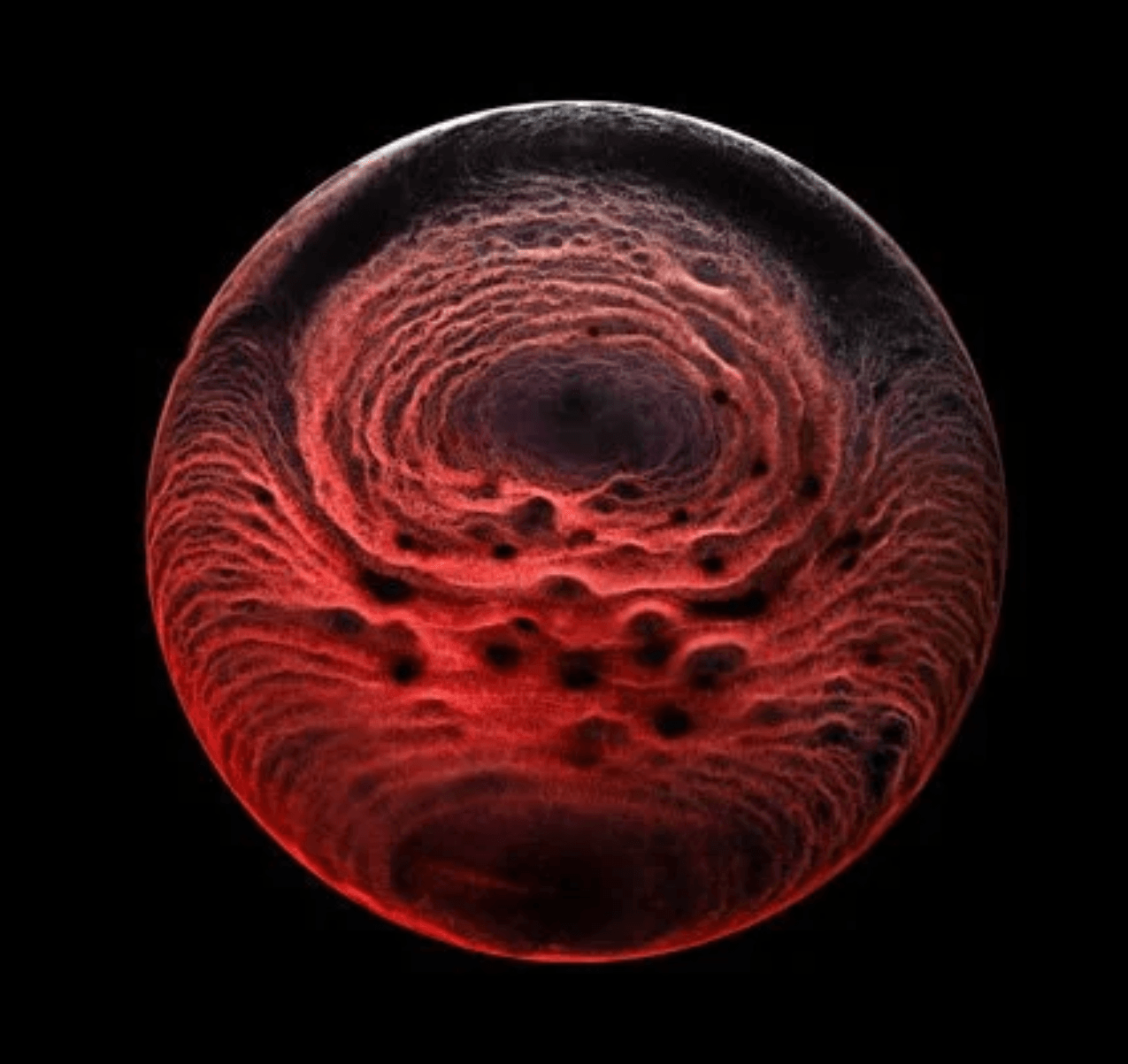
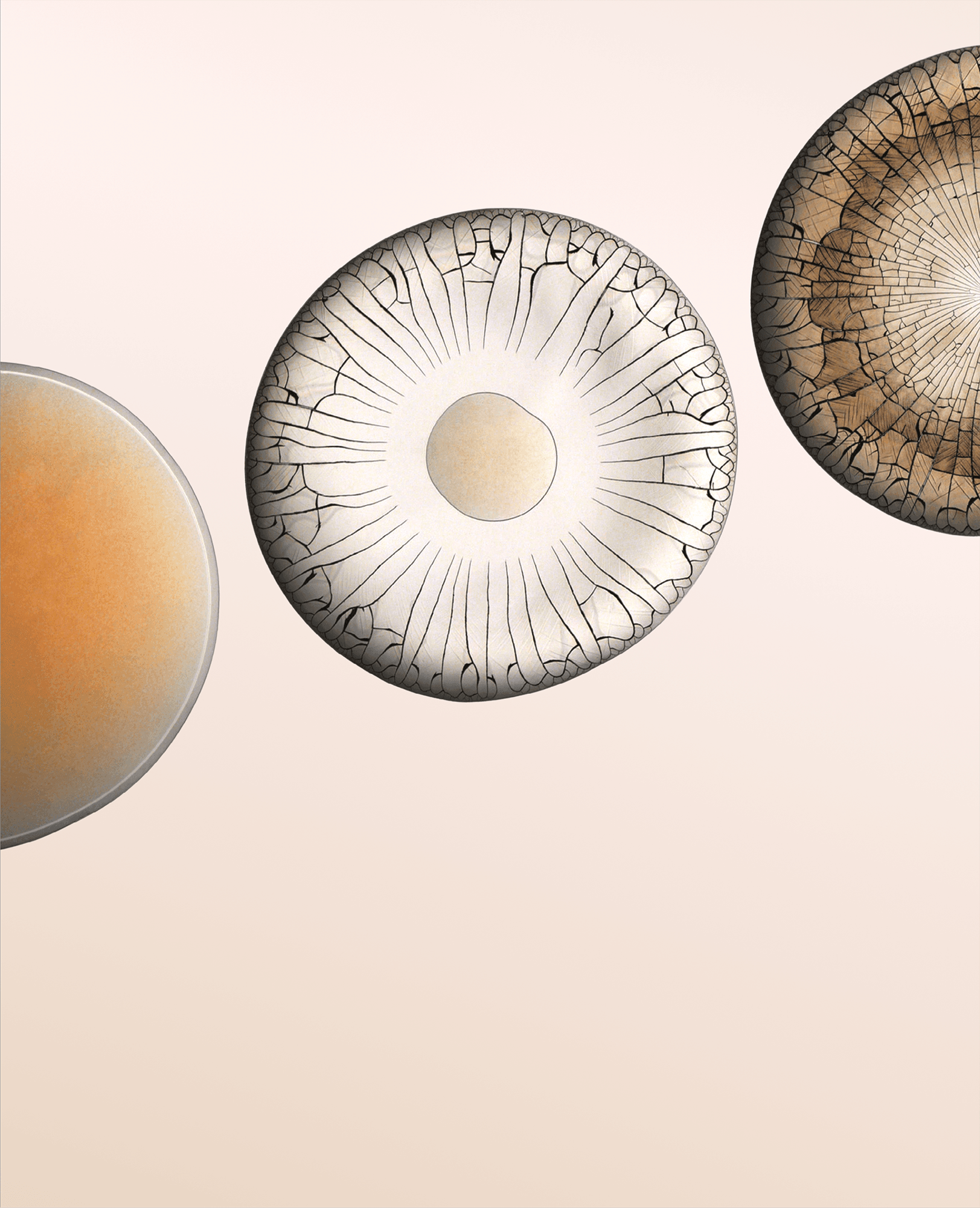Photographer Ernie Button explores the stains left behind when various liquors evaporate. This one comes from a single malt scotch whisky by The Balvenie. The stain itself is made up of particles left behind when the alcohol and water in the whisky evaporate. The pattern itself depends on a careful interplay between surface tension, evaporation, pinning forces, and internal convection as the whisky puddle dries out. (Image credit: E. Button/CUPOTY; via Colossal)
Tag: deposition

Vanishing Spirits: Rice-Based Whisky
In yesterday’s post, photographer Ernie Button showed us that barrel-aged gin can leave behind an evaporation pattern remarkably similar to scotch whisky. But even among whiskys not every spirit uses the same grains.
Here we see patterns left behind by a 10-year-aged, rice-based whisky. The stains are entirely different than those of (barley-based) scotch. The rice leaves behind stains with distinct regions, including a radially uniform rim and an interior reminiscent of satellite photos. Presumably the interaction of rice and the cask leaves the whisky with surfactants and polymers that behave rather differently than those of scotch.
It takes time for spirits to take on character from the casks they’re aged in. Tomorrow we’ll take a look at just how much aging is necessary for scotch’s patterns to emerge. (Image, research, and submission credit: E. Button; see also)

Vanishing Spirits: Gin
Photographer Ernie Button has spent years exploring the patterns left by evaporating scotch. A team of researchers found that the uniformity of scotch whisky’s stain requires three ingredients: alcohol to drive concentration gradients, surfactants to pull particulates away from the drop’s edge, and polymers to help stick particles to the glass.
Button wondered whether other spirits might produce similar patterns, and, indeed, some do. The photos above are stains left behind by evaporated gin that’s been aged for a year in oak casks. The patterns are extremely similar in appearance to those from aged scotch whiskies, suggesting that the same fluid dynamical effects are at play here, despite the difference in liquor. But do all grain spirits make these patterns? Check back tomorrow to find out. (Image, research, and submission credit: E. Button; see also)

Blooming Deposits
Evaporate a droplet full of silica nanoparticles, and you’ll get beautiful, flower-like films. As the water evaporates, dry nanoparticles build up in a solid deposit. The evaporation creates a pressure gradient that pulls toward the center of the drop, forcing the deposit to bend. As stress builds in the deposit, cracks form petal-like segments. The number of cracks is indicative of how much of the drop was solid material; the higher the volume fraction of particles is, the fewer cracks form and the less the deposit bends. (Image, video, and research credit: P. Lilin et al.)

Sublimation
Sublimation is a transition directly from a solid phase to a gaseous one. Given typical Earth atmospheric conditions, one of the most commonly observed examples of sublimation is that of solid carbon dioxide, a.k.a. dry ice. Submerging dry ice in water both speeds up the sublimation–since water is a better conductor of heat than air–and creates ethereal fog that’s a combination of the expanding carbon dioxide and condensate from the water. This gorgeous video from Wryfield Lab lets you admire the process close-up. As the dry ice sublimates, watch for the ice crystals that grow on its surface. This is deposition–the opposite of sublimation–and comes from water vapor freezing onto the dry ice. (Video credit: Wryfield Lab; via Gizmodo)
A warning for those who want to try this at home: only do this in well-ventilated spaces. The shift from solid to gas requires a huge increase in volume. Carbon dioxide is denser than air, so it does stay low to the ground, but you can still suffocate yourself (or children or pets) if you do this in an enclosed space.

Self-Assembly via Evaporation
When working at the microscale, engineering structures like those used for drug delivery systems requires ingenuity. Since it isn’t possible to manipulate particles manually, researchers harness physical effects to do the work for them. Here a droplet filled with millions of polystyrene microparticles sits on a hydrophobic surface, which helps keep the drop’s spherical shape. As the drop evaporates, surface tension and internal flow in the drop help the microparticles self-assemble into a microscopic soccer-ball-like shape. (Video credit: A. Marin et al.; submission by A. Marin)

Reader Question: Frosty Cars
Reader Mike L asks:
Why do I never see frost on my car when I park in a detached garage or under a carport?Great question! Frost forms on surfaces when their temperature drops below the freezing point of water and the dew point of the surrounding air. The water vapor in the air gets deposited as a solid directly; this is called deposition. This means that the surface–in this case your car–has to be colder than the nearby air. Neither conduction nor convection of heat between your car and the surrounding air can cause this drop; heat transfer between your car and the surrounding air would tend to make them the same temperature, not make the car colder than the air. The third–and typically least effective–type of heat transfer, radiation, is the answer because it allows heat transfer between two objects that are not in direct contact like the air and car are.
Frost typically forms on still, clear nights with little clouds or wind. A car sitting beneath a clear night sky will radiate heat out into space. Since space is much, much colder than the air, this radiation cooling to space allows the car’s surface temperature to drop below that of the surrounding air, which is not a good radiator by comparison. On a night with little wind (and thus little convection), this radiation cooling can be quite effective. Frost will tend not to form on one’s car under a carport because the car is sheltered from the night sky, blocking such radiative cooling. Having a tree or house blocking the car from the night sky is also effective at preventing frost formation. (Photo credit: N. Sharp; with thanks to Keri B and Jerry N for the meteorological assistance)

How Coffee Rings Form
Coffee rings (an ubiquitous feature of academia) are formed by the deposition of particles as the liquid evaporates. When a coffee drop evaporates, capillary action draws the coffee particles toward the edges of the drop, where they congregate into a ring. Research now suggests that this is due to the spherical nature of the particles. Ellipsoidal particles, in contrast, clump together and result in a uniform stain once their carrier liquid evaporates. The effect seems to be due to the particles’ effects on surface tension; the ellipsoidal particles deform the surface of the droplet as it evaporates such that they are not pulled to the edges. Adding a surfactant, like soap, that decreases surface tension caused the ellipsoidal particles to form rings just as the spherical particles do. (submitted by Neil K) #