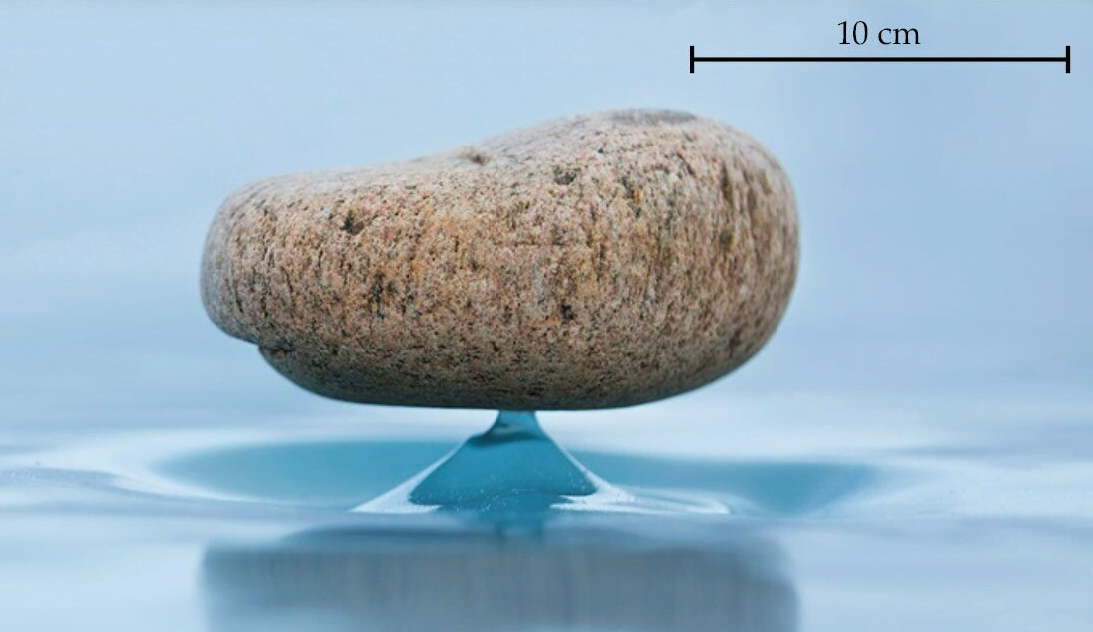
On Lake Baikal, where Siberian winters are long and cold but have little precipitation, you can find a strange phenomenon: stones that balance on a thin spire of ice. Known as Zen stones — thanks to their visual similarity to stacks of balanced stones in Japanese Zen gardens — these natural oddities rely on time and sublimation, a transition from ice to vapor without melting.
The process is simple. Toss a stone on the ice and wait. As the sun shines, the ice will sublimate, transforming from ice directly to vapor at an estimated rate of ~2 mm per day, for Lake Baikal’s typical weather. But the stone’s presence acts like an umbrella, protecting some of the ice beneath it from the sunlight that is critical for sublimation. As a result of this umbrella effect, a thin column of ice remains beneath the stone.
In the lab, researchers were able to recreate the process in less time by tweaking the temperature, humidity, and irradiance to enhance sublimation. Instead of stones, they used metal disks, but their Zen stones made their ice columns just the same. (Image and research credit: N. Taberlet and N. Plihon; via Physics Today)