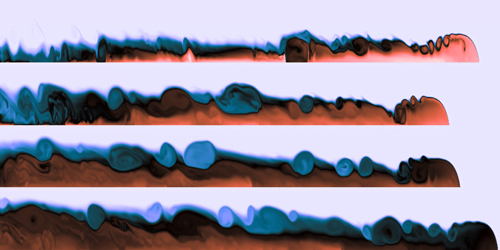
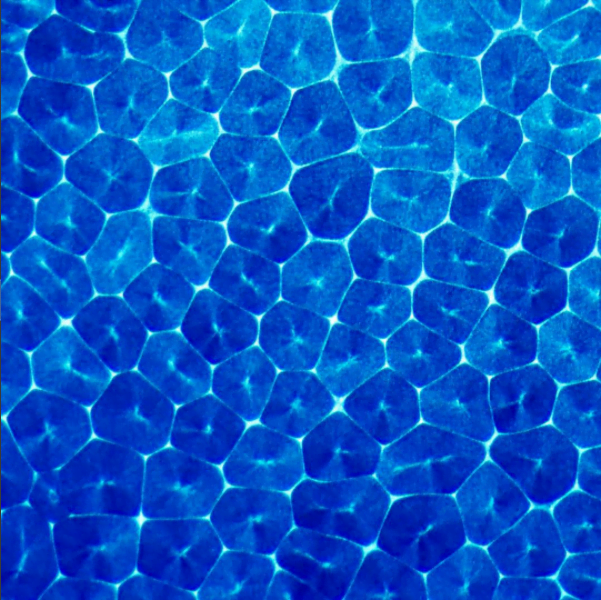
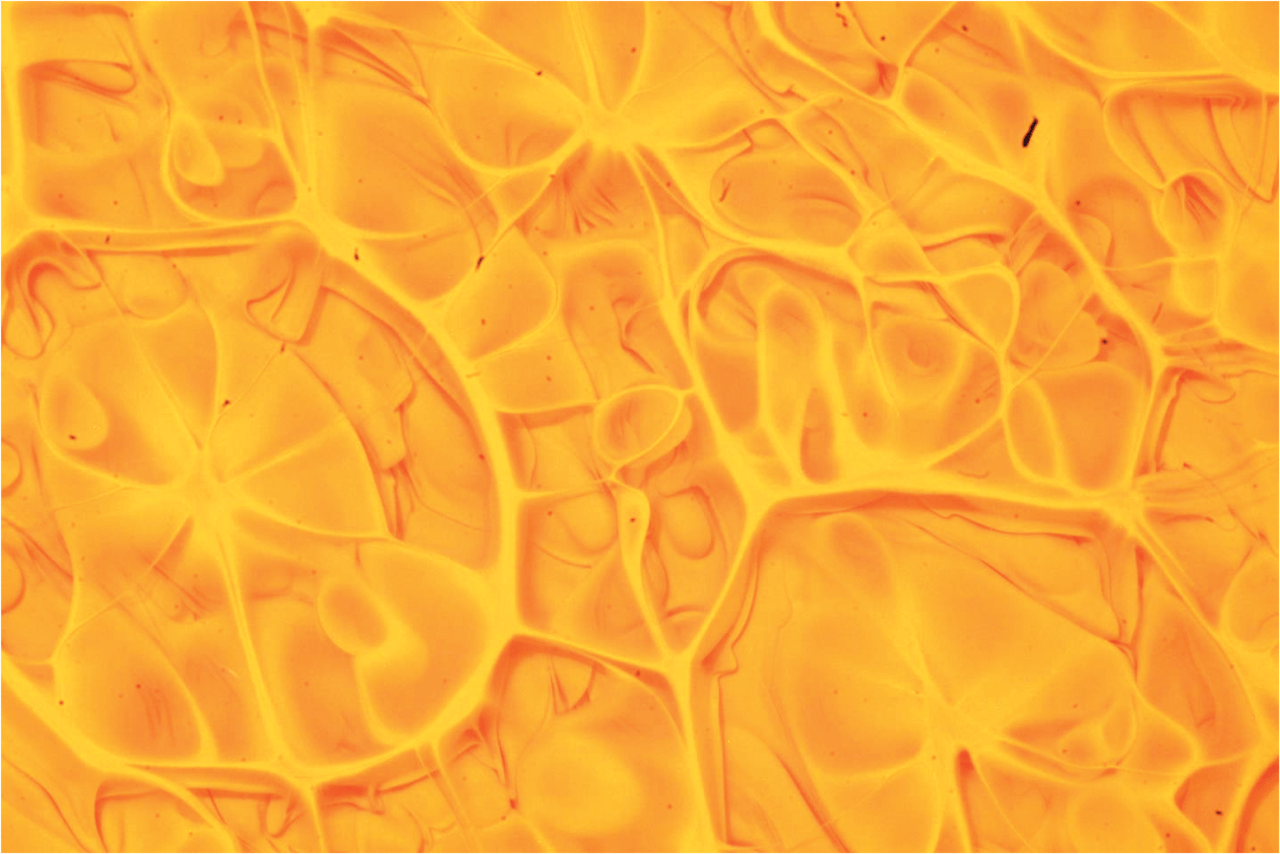
Steve Mould opens this video with a classic physics toy that uses materials of different densities as a brainteaser. Two transparent, immiscible liquids fill the container, along with beads of a couple different densities. When you shake the toy, the liquids emulsify, creating a layer with an intermediate density. As the two liquids separate, the emulsified middle layer disappears, causing the beads (which have densities between that of the two original liquids) to come together.
The rest of the video describes the challenges of expanding this set-up into three immiscible liquids and four sets of beads. Along the way, Steve had to contend with issues of miscibility, refractive index, and even chemical solvents. It’s amazing, sometimes, what it takes to make a seemingly simple idea into reality. (Video and image credit: S. Mould)