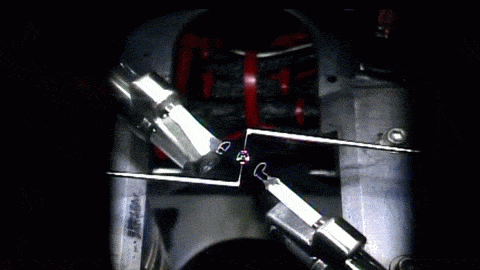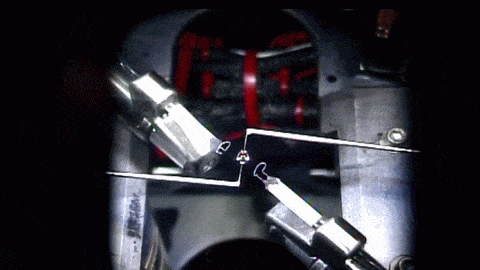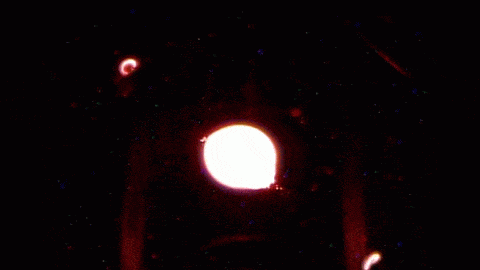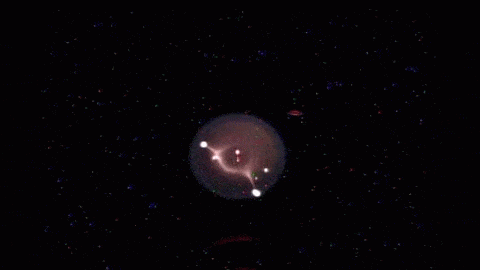
The jellyfish-like light show in the animations above shows the life and death of a flame in microgravity. The work is part of the Flame Extinguishment Experiment 2 (FLEX-2) currently flying aboard the International Space Station. When ignited, the fuel droplet creates a blue spherical shell of flame about 15 mm in diameter. The spherical shape is typical of flames in microgravity; on Earth, flames are shaped like teardrops due to the effects of buoyancy, which exists only in a gravitational field. The bright yellow spots and streaks that appear after ignition are soot, which consists mainly of hot-burning carbon. The uneven distribution of soot is what causes the pulsating bursts seen in the middle animation. When soot products drift back onto the fuel droplet, it causes uneven burning and flame pulses. The final burst of flame in the last animation is the soot igniting and extinguishing the flame. Fires are a major hazard in microgravity, where oxygen supplies are limited and evacuating is not always an option. Scientists hope that experiments like FLEX-2 will shed light on how fires spread and can be fought aboard spacecraft. For more, check out NASA’s ScienceCast on microgravity flames. (Image credits: NASA, source video; submitted by jshoer)
Tag: combustion

Supernova Explosion
Type 1a supernovae occur in binary star systems where a dense white dwarf star accretes matter from its companion star. As the dwarf star gains mass, it approaches the limit where electron degeneracy pressure can no longer oppose the gravitational force of its mass. Carbon fusion in the white dwarf ignites a flame front, creating isolated bubbles of burning fluid inside the star. As these bubbles burn, they rise due to buoyancy and are sheared and deformed by the neighboring matter. The animation above is a visualization of temperature from a simulation of one of these burning buoyant bubbles. After the initial ignition, instabilities form rapidly on the expanding flame front and it quickly becomes turbulent. (Image credit: A. Aspden and J. Bell; GIF credit: fruitsoftheweb, source video; via freshphotons)

Lighting a Match
The schlieren optical technique is ideal for visualizing differences in fluid density and is an important tool for revealing flows humans cannot see with their naked eyes. In this high speed video, a professor lights a match. The initial strike generates friction and heat sufficient to convert some of the red phosphorus in the match head to its more volatile white phosphorus form. We see this in the schlieren as the cloud-like burst in the first several seconds. The heat from the phosphorus combustion ignites the sulfur fuel and potassium chlorate oxidizer in the match head to create a more sustained flame. During this period, wavy, smoke-like whorls of hot air rise from around the flame as buoyancy takes over. The upward movement of hot air draws in cooler air from the surroundings, providing the flame with an ongoing source of oxygen and allowing it to grow. (Video credit: RMIT University)

Inside a Rocket
Rockets often utilize liquid propellants for their combustion. To maximize the efficiency during burning, the liquid fuel and oxidizer must mix quickly and break up into an easily vaporized spray. One method to achieve this is to inject the fuel and oxidizer as liquid jets that collide with one another. For high enough flow rates, this creates a highly unstable liquid sheet that quickly atomizes into a spray of droplets. The animation above shows an example of two impinging jets, but rockets using this method would typically have more than just two injection points. Other rockets use co-axial or centrifugal injectors to mix and atomize the fuel and oxidizer prior to combustion. (Image credit: C. Inoue; full-scale GIF)

Balloon Explosion
These photos are shadowgraphs of a hydrogen flame exploding inside a balloon. The shadowgraph optical technique highlights density and temperature variations through their effect on a fluid’s refractive index. Here we see that the hydrogen flame has a strong cellular structure and is more turbulent than a methane flame. The cellular structure is a sign of an instability in the curved flame front. The instability and accompanying cellular appearance are a result of the complicated transport and reaction of fuel and oxidizer inside the flame. (Photo credits: P. Julien et al.)

Putting Out Wildfires Using Explosives

Wildfires damage millions of acres of land per year in the United States alone. Using explosives to put out an uncontrolled wildfire sounds a bit crazy, but it’s actually not that far-fetched. The animations above are taken from high-speed footage of a propane fire interacting with a blast wave. The first animation shows what the human eye would see, and the second is a shadowgraph video, a technique which highlights differences in density and makes the flame’s convection and the blast wave itself visible. At close range, the shock wave from the explosion and the high-speed gas behind it push the flames away from their fuel source, stopping combustion almost immediately. For a flame farther away from the blast, the shock wave introduces turbulent disturbances that can destabilize the flame. Much work remains to be done before the technique could be scaled from the laboratory to the field, but it is an exciting concept. You can read more about the work here. (Research credit: G. Doig/UNSW Australia; original videos: here and here; submitted by @CraigOverend)

Rubens’ Table
Veritasium’s new video has an awesome demonstration featuring acoustics, standing waves, and combustion. It’s a two-dimensional take on the classic Rubens’ tube concept in which flammable gas is introduced into a chamber with a series of holes drilled across the top. Igniting the gas produces an array of flames, which is not especially interesting in itself, until a sound is added. When a note is played in the tube, the gas inside vibrates and, with the right geometry and frequency, can resonate, forming standing waves. The motion of the gas and the shape of the acoustic waves is visible in the flames. Extended into two-dimensions, this creates some very cool effects. (Video credit: Veritasium; via Ryan A.; submitted by jshoer)

“Aurora”
This bulbous, ethereal shape is a spreading flame front captured by artist Fabian Oefner in his new “Aurora” series. Oefner used a few alcohol droplets in a glass vessel and ignited the volatile vapors, capturing the propagating flame. Take a look at it in action. Because the air inside the vessel is mostly still, the chemical reactions in the combustion occur much faster than the air’s motion. As a result, the flame spreads as a thin sheet instead of a uniform, widespread flame. The wrinkled and corrugated look of the flame front is due local turbulence distorting the flame. (Photo credit: F. Oefner)

Holiday Fluids: What is Fire?
Snowy holidays and long, dark nights are a great time to sit by the fire or enjoy some candlelight. We’ve talked before about how buoyancy affects a flame’s shape, how atomization mixes liquid fuel and oxidizers, how flames propagate, how internal combustion works and how instabilities can end combustion. But in all that we haven’t addressed what fire actually is! Combustion is a chemical process–a reaction between a hydrocarbon fuel and oxygen, but the flame we’re accustomed to seeing is a combination of blue light produced by the complete reaction and incandescent red/orange/yellow light from glowing soot particles produced when there is insufficient oxygen for the reaction. If you have time after the Minute Physics version, this video from Ben Ames has a wonderful explanation of flames. Of course, if you just prefer your holiday fun with more explosive high-speed videos, you’re going to want to see this Christmas tree made from detonation cord (see 2:40 for the start of the best part). This wraps up our holiday-themed fluid dynamics series. Happy holidays from FYFD! (Video credit: Minute Physics)

Fire in Microgravity
In the movie “Gravity” Sandra Bullock’s character battles a fire aboard the International Space Station. Combustion is a huge concern in space habitats. Microgravity fires are challenging to detect and fight because they behave very differently in the absence of buoyancy. On Earth, buoyancy makes hot air rise from a flame while cooler air is pulled in near the base. This feeds fresh oxygen to the teardrop-shaped flame. In space, there is no buoyancy and flames are spherical. They also burn at lower temperatures and lower oxygen concentrations–so low, in fact, that the oxygen depletion necessary to extinguish a fire is lower than what humans require to survive.
No buoyancy makes it harder for fires to spread, but it also makes them harder to detect since smoke doesn’t rise toward a detector on the ceiling. Instead, fire detectors aboard the Space Station are housed in the ventilation system that moves air through the modules constantly. In the event of a fire, astronauts use a three-step fire suppression system. First, they shut off the ventilation system to delay the fire’s spread. Then they shut off power to the affected unit, and, finally, they use fire extinguishers on the flames. The Russian module is equipped with a foam extinguisher and the others use CO2 units. (Image credit: Warner Brothers)