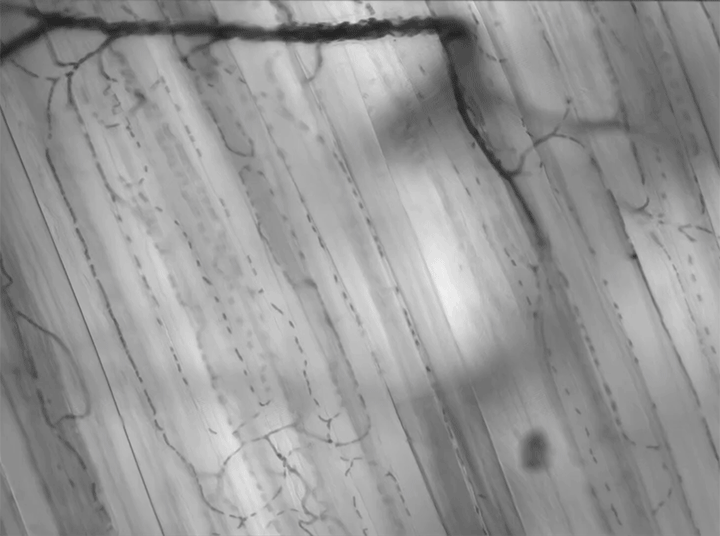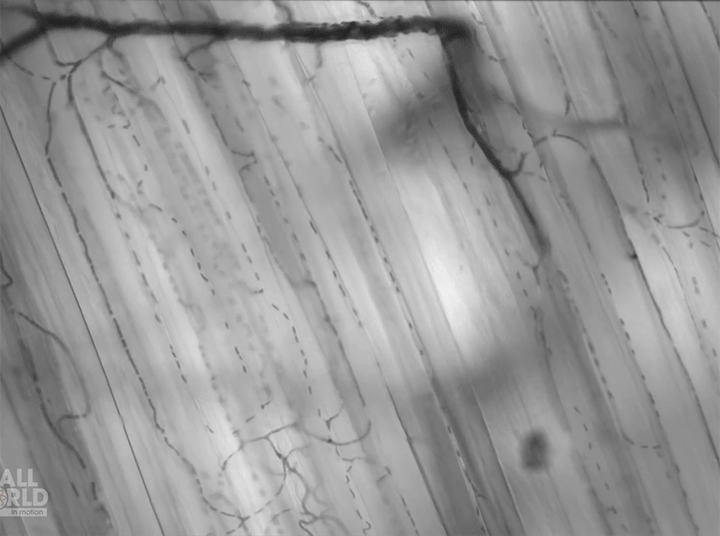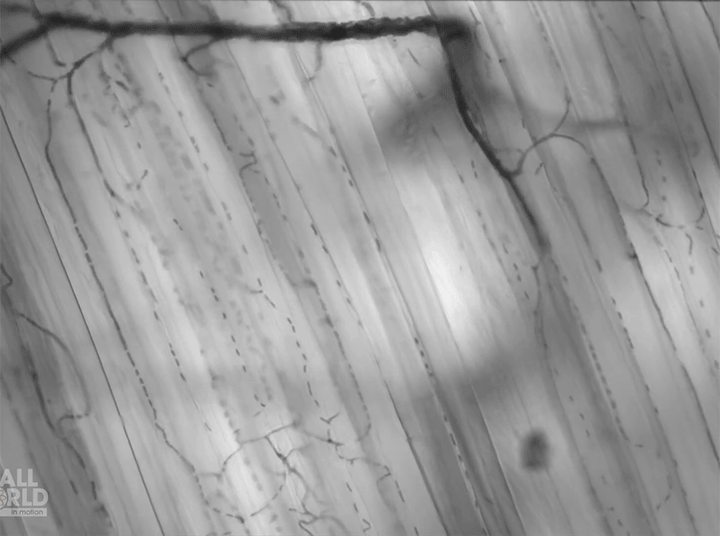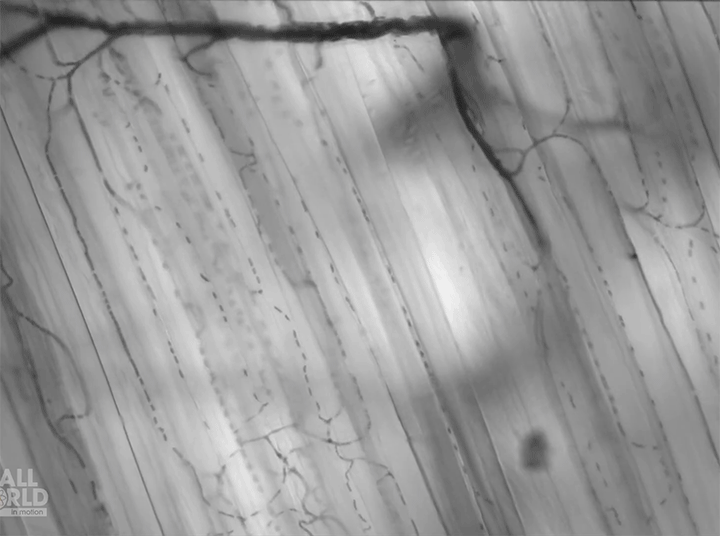
Today’s video shows red blood cells flowing through a capillary network in a rat’s skeletal muscle. At this resolution, our eyes can follow the paths of individual red blood cells squeezing through each capillary, as well as the faster blur of thicker capillaries where many cells can pass at once. Watching videos like this is a great way to build intuition for particle image velocimetry, streaklines, and other flow visualization methods as our brains can readily recognize where the cells are moving fast and where they are slower. (Video and image credit: Dr. G. McEvoy et al.; via Colossal)
Tag: blood flow

Blood Flow in a Fin
This award-winning video shows blood flowing through the tail fin of a small fish. Cells flow outward in a central vessel, then split to either side for the return journey. In this microscopic video, the speed of individual cells seems quite fast, even though the vessels themselves are only wide enough for the blood cells to move in single file. Flow at the microscale can be counterintuitive like that. (Video and image credit: F. Weston for the 2023 Nikon Small World in Motion Competition; via Colossal)


Placental Fluid Dynamics
The placenta, critical as it is to human life and development, is likely the least-studied organ in the body. Reasons for that abound, from the ethics of studying pregnant people to the historical marginalization of female bodies in medical studies. But what little we do know shows that the placenta is quite incredible.
Shaped somewhat like a flattened cake, the placenta contains a tangle of fetal blood vessels — an estimated 550 kilometers’ worth — bathed in maternal blood. The enormous surface area — nearly 13 meters squared — enables the exchange of oxygen, glucose, and urea through diffusion. These exchanges don’t take place in still conditions, though; blood is always flowing through the vessel network. This means that each exchange depends on both the speed of diffusion and the speed of the flow, a relationship that’s captured with the dimensionless Damköhler number.

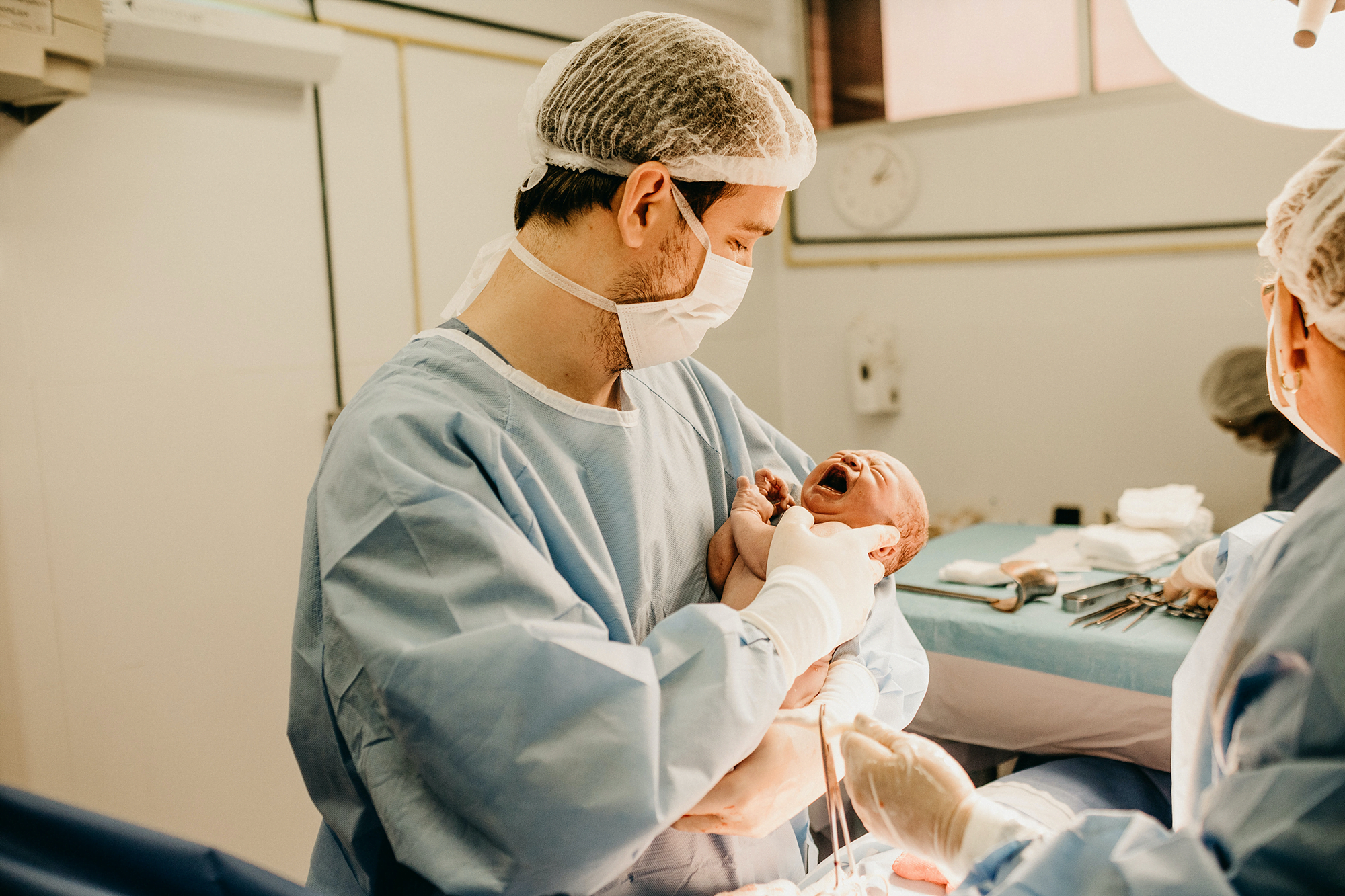
Illustration of the intertwined blood vessels of the placenta. Some exchanges, like carbon monoxide and glucose, are diffusion-limited, meaning that increased blood flow cannot speed up the process (though additional blood vessel surface area could). In contrast, carbon dioxide and urea are flow-limited exchanges. Fascinatingly, oxygen is close to being both diffusion- and flow-limited, suggesting that the organ has optimized for this exchange. Since pregnancy also involves a large increase in maternal blood volume and changes in lung capacity to help provide oxygen, it seems like the pregnant body heavily emphasizes delivering oxygen to the developing fetus. (Image credit: newborn – J. Borba, placenta – iStock/Sakurra; via Physics World; submitted by Kam-Yung Soh)

A Primer on Blood Pressure
Some of the most important fluid dynamics goes on every moment inside our bodies. After only a few weeks of gestation, the human heart begins its lifelong task of pumping blood throughout tens of thousands of kilometers’ worth of blood vessels. One of our simplest methods for tracking the health of this critical system is a person’s blood pressure, which measures the forces exerted on our blood vessels as our hearts pump. This video gives a brief primer on blood pressure as well as some of the problems that arise when extended bouts of high blood pressure damage our blood vessels. (Image and video credit: TED-Ed)

New Signs of Turbulence in Blood Flow
Our bodies are filled with a network of blood vessels responsible for keeping our cells oxygenated and carrying away waste products. In many ways, our blood vessels are tiny pipes, but there’s a crucial difference in the flow they carry: it’s pulsatile. Because the flow is driven by our hearts, rather than a continuous pump, every heartbeat creates a distinct cycle of acceleration and deceleration in the flow. And new research has found that this cycle, when combined with curvature or flow restrictions like plaque build-up, can create turbulence in unexpected places.
Specifically, the researchers found that decelerating pipe flows can develop a helical instability that breaks down into turbulence, even in vessels where purely laminar flow would be expected. In the animations above, you can see the flow slow, develop swirls and then break into turbulence. The flow becomes laminar again as it accelerates, but during that brief bout of turbulence there’s much higher forces on the walls of a blood vessel. Over time, that extra force could contribute to inflammation or even hardening of the arteries. (Image and research credit: D. Xu et al.; via phys.org)

Flow in the Heart
Few flows are more integral to our well-being than blood flow through the heart. Over the course of our lives, our hearts develop from a few cells pushing viscous blood through tiny arteries to the muscular center of a vast circulatory network, capable of powering us through incredible physical feats. What’s most astonishing about all this is that the heart goes through all these changes and adaptations without ever pausing.
Peering into the heart to see it in action is difficult, but researchers today are combining imaging techniques like CT and MRI with computational fluid dynamics to build patient-specific heart models. Not only does this help us understand hearts in general; it’s paving the way toward predicting how a specific treatment may affect a patient. Imagine, for example, being able to simulate and compare different models of an artificial heart valve to see which will work best for a particular patient. We’re not to the point of doing so yet, but it’s a very real possibility in the future.
To see some examples of predicted and measured heart flows, check out this video by J. Lantz. In the meantime, happy Valentine’s Day! (Image credits: Linköping University Cardiovascular Magnetic Resonance Group, video source; via Another Fine Mesh)

Using Embolisms to Fight Cancer
Blocking blood vessels by creating embolisms is, under most circumstances, very bad. But researchers are exploring ways to fight cancer by intentionally and strategically creating these blockages. In gas embolotherapy, researchers inject fluid droplets, which can carry chemotherapy drugs, into the bloodstream. Once they circulate into a cancerous tumor, they use ultrasound to vaporize the droplet and create a gas bubble. Those bubbles lodge inside the capillaries of the tumor, starving it of fresh blood and trapping the chemotherapy drugs inside. It’s a one-two punch to the cancer. Without blood flow, the cancer cells die, and, since the cancer-killing drugs get mostly trapped inside the tumor, patients may require lower dosages and endure fewer side effects. The technique is currently in animal testing, but hopefully it will be a valuable therapy for human patients in the future. (Image credit: Chemical & Engineering News; research credit: Y. Feng et al.; via AIP)

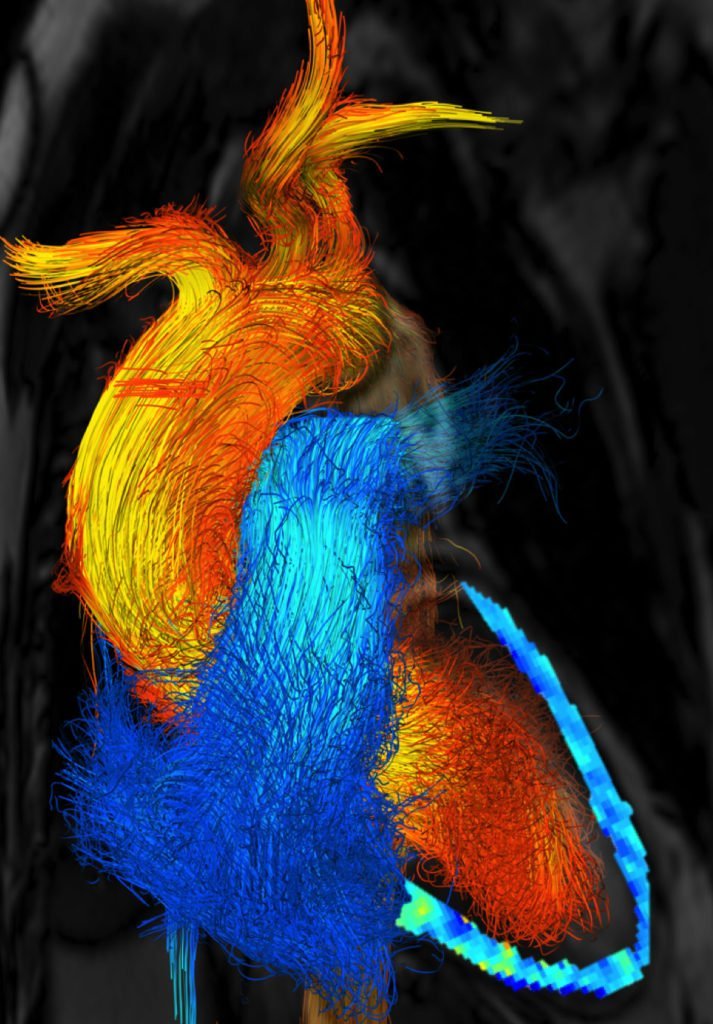
Flow Inside the Heart
Inside each of us is a remarkable and constant flow, driven by a muscle that’s always at work. As blood circulates through our bodies, it goes through a surprisingly varied journey. In the heart, as seen above, blood flow is very unsteady and quite turbulent, due to the beating pulse of the heart. As valves open and close and the muscle walls constrict and relax, the rushing blood moves in eddy-filled spurts. In the outer reaches of our capillaries, however, the nature of the flow is quite different. Thanks to smaller vessel sizes and other factors, capillary blood flow is much steadier and more laminar. Viscosity becomes more important, as do the non-Newtonian properties of components in our blood. (Image credit: mushin111/YouTube, source; via Science; submitted by Gary N.)

Blood Flow Simulations
Though we may not often consider it, our bodies are full of fluid dynamics. Blood flow is a prime example, and, in this video, researchers describe their simulations of flow through the left side of the heart. Beginning with 3D medical imaging of a patient’s heart, they construct a computational domain – a meshed virtual heart that imitates the shape and movements of the real heart. Then, after solving the governing equations with an additional model for turbulence, the researchers can observe flow inside a beating heart. Each cycle consists of two phases. In the first, oxygenated blood fills the ventricle from the atrium. This injection of fresh blood generates a vortex ring. Near the end of this phase, the blood mixes strongly and appears to be mildly turbulent. In the second phase, the ventricle contracts, ejecting the blood out into the body and drawing freshly oxygenated blood into the atrium. (Video credit: C. Chnafa et al.)