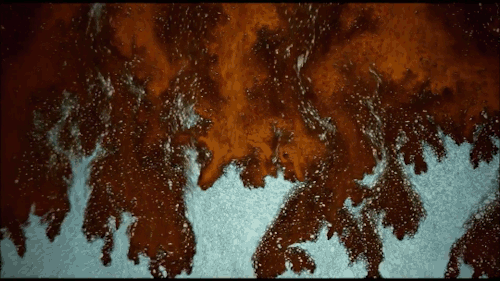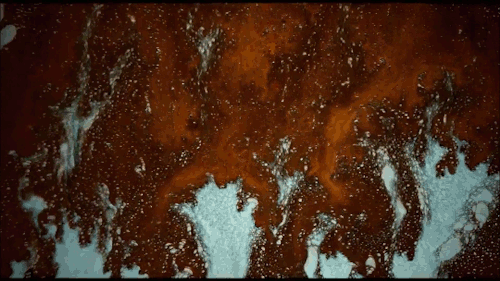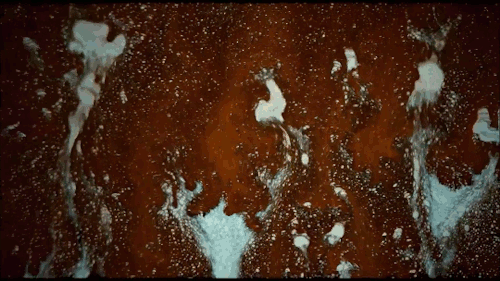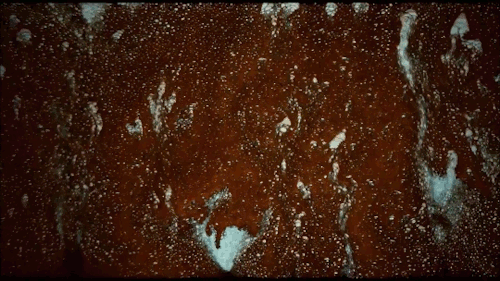
Thomas Blanchard is back with another beautiful music video. This one features ink cascading over various shapes underwater. Lots of tiny mushroom-shaped Rayleigh-Taylor instabilities here caused by the ink’s greater density compared to the surrounding water. There are also some lovely examples of transitional flow, especially around the spheres. Initially, flow over the spheres looks completely smooth and laminar. But, on the latter half of the sphere, where the flow is under increasing pressure, you see disturbances growing until little fingers of ink break away entirely. Be sure to watch the whole video; you don’t want to miss this! (Video and image credit: T. Blanchard)
Tag: Rayleigh-Taylor instability

Convection Without Heat
We typically think of convection in terms of temperature differences, but the real driver is density. In the animations above, cream sitting atop a liqueur is undergoing solutal convection – no temperature difference needed! The alcohol in the liqueur mixes with the cream to form a lighter mixture that rises to the surface. The lower surface tension of the alcohol is also good at breaking up the cream, forming little cells. As the alcohol in those cells evaporates, the cream gets heavier and sinks down into the liqueur, where it can pick up more alcohol, rise back to the surface, and begin the cycle again. (Image credit: J. Monahan et al., source)

Breaking With a Wave
For rocket combustion and other applications, like watering your lawn with a hose, a stream of fluid may need to be broken up into droplets. While simply spraying a liquid jet will make it break up, waving that jet back and forth will break it up faster. A recent study simulated this problem numerically to determine the exact mechanisms driving that break-up. The researchers found two major culprits.
The first is a Kelvin-Helmholtz, or shear-based, instability. When a jet leaves the nozzle, there’s friction between it and the comparatively still air surrounding it. This creates tiny ripples in the surface that eventually grow into the distortions we can see, and it’s found in all jets, regardless of their side-to-side motion.
The second culprit, which is only found in the oscillating jet, is a Rayleigh-Taylor instability. By moving the jet side-to-side, you’re driving the dense liquid into less dense air, which creates a different set of disturbances that also help break up the jet. The final result: swinging the jet side-to-side breaks it into smaller droplets faster. (Image and research credit: S. Schmidt et al.)

“Water Ballet”
Artist Kamiel Rongen uses common substances like paint, oil, eggs, and even air freshener to create what he calls “water ballet.” His videos are full of ethereal and surreal landscapes full of color and motion. Buoyancy (or the lack thereof) plays a major role in his work – fluids often spurt upward like alien creatures emerging from a chrysalis. I’ve been debating with myself whether the fluids are actually rising or if they’re falling in front of an upside-down camera, and I’m not completely certain either way! I think that’s a testament both to Rongen’s artistry and to the awesome physics involved. Check out the full video below and you can see many more examples of Rongen’s work on his website. (Image and video credit: K. Rongen; h/t to James H.)

Dissolving Candy
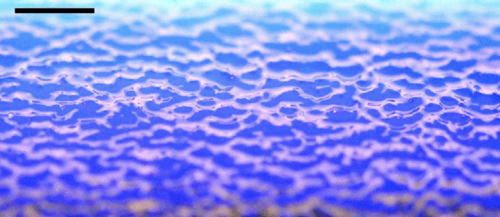
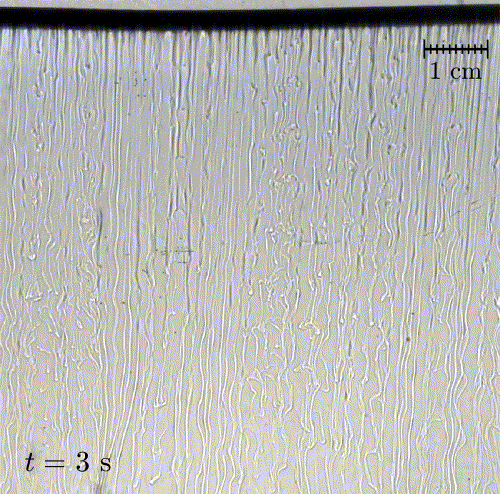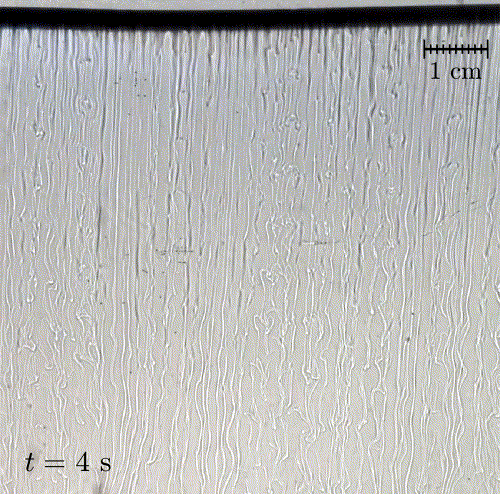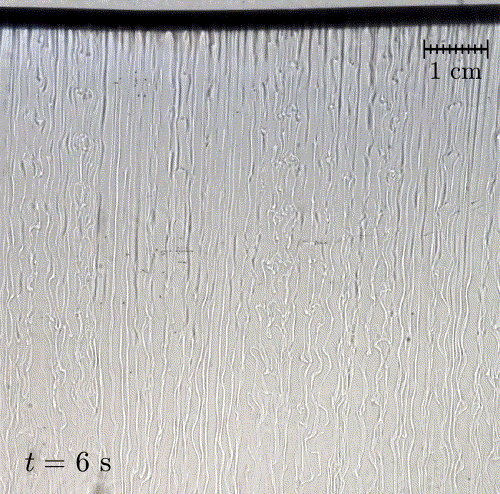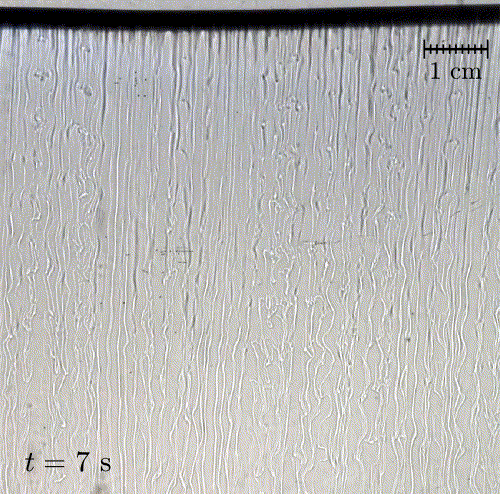
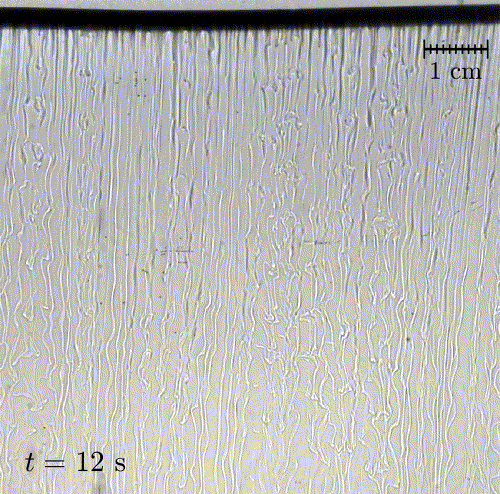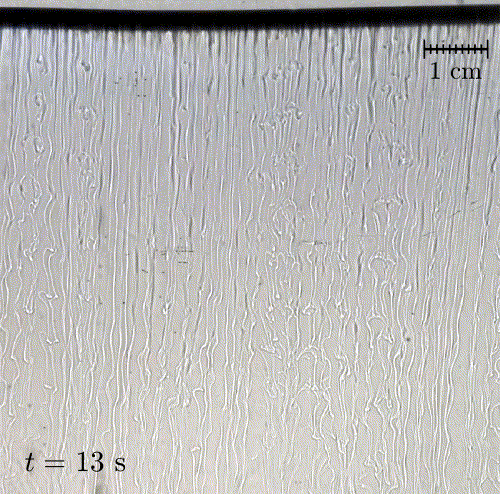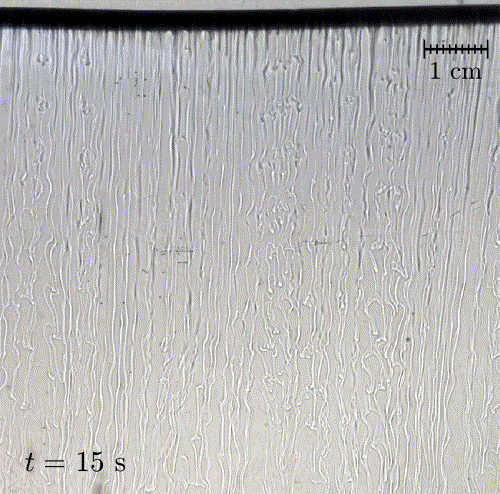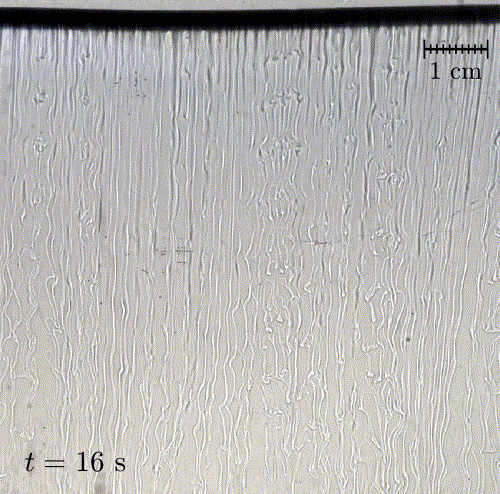
In nature, solid surfaces often evolve over time in conjunction with the flows around them. This is how stalactites, canyons, and hoodoos all form and change over time. Here researchers examine a surface formed from hard candy that is dissolving from below. Over time, the initially flat surface develops a pitted appearance (top image, scale bar is 1 cm) with roughness that is approximately 1 mm in scale. Flow visualization (bottom row) suggests that these pits result from local flow where narrow, millimeter-sized dense plumes fall away from the surface.
As material dissolves from the candy, it forms a dense layer of sugar-water mixture near the solid surface. Once that layer grows to a critical thickness, it will be too unstable for viscosity to counter. At that point, the Rayleigh-Taylor instability takes over, causing the dense sugar-water layer to break up into narrow, sinking plumes. Although each area is evolving independently, the rate at which material dissolves is uniform everywhere, so the dissolving body retains the same shape over time. (Image and research credit: M. Davies Wykes et al., source)

Impressionist Foams
Imagine taking two panes of glass and setting them in a frame with a small gap between them. Then partially fill the gap with a mixture of dye, glycerol, water, and soap. After turning the frame over several times, the half of the frame will be filled with foamy bubbles. When you flip it again, the dyed glycerol-water will sink and penetrate the bubble layer, creating complex and beautiful patterns as it mixes. Some of the bubbles may get squeezed together until they coalesce into larger bubbles that shoot upward thanks to their increased buoyancy. Other smaller bubbles will wend their way upward as neighboring fluid shifts. If you examine the tracks left by individual bubbles, you can find patterns reminiscent of Impressionist paintings, as seen at the end of this Gallery of Fluid Motion video. (Image credit: A. Al Brahim et al., source)

“Ink in Motion”
In this short film, the Macro Room team plays with the diffusion of ink in water and its interaction with various shapes. Injecting ink with a syringe results in a beautiful, billowing turbulent plume. By fiddling with the playback time, the video really highlights some of the neat instabilities the ink goes through before it mixes. Note how the yellow ink at 1:12 breaks into jellyfish-like shapes with tentacles that sprout more ink; that’s a classic form of the Rayleigh-Taylor instability, driven by the higher density ink sinking through the lower density water. Ink’s higher density is what drives the ink-falls flowing down the flowers in the final segment, too. Definitely take a couple minutes to watch the full video. (Image and video credit: Macro Room; via James H./Flow Vis)


Acrylic and Oil
Photographer Alberto Seveso is well-known for ink in water art, some of which FYFD has featured previously (1, 2, 3). More recently, he’s been experimenting with alternative methods, dropping fluids like acrylic paint into sunflower oil. The effect is quite different but no less beautiful. Because the paint and oil are immiscible, the boundaries between the two fluids are much more clearly defined and highlighted in an iridescent sheen. Instead of appearing like billowing waves of silk, the paint forms abstract and alien shapes driven by gravity, inertia, and density differences. For many more great examples, check out Seveso’s website. (Photo credit: A. Seveso)

Four Seasons
The team behind Beauty of Science decided to explore the four seasons in this video combining macro footage of crystal growth, chemical reactions, and fluid dynamics. It’s always a fun game with videos like this to try and guess exactly what makes the mesmerizing patterns we see. Are those blue streaming waves in Spring caused by alcohol shifting the surface tension in a mixture? Are the dots of color welling up in Autumn a lighter fluid bursting up from underneath a denser one? As fun as the visuals are, though, what really made this video stand out for me was its excellent use of “The Blue Danube” to tie everything together. Check it out and don’t forget the audio! (Video credit: Beauty of Science; via Gizmodo)





Accidental Painting

Some paintings of Mexican artist David Alfaro Siqueiros feature patchy, spotted areas of contrasting color formed by what Siqueiros described as “accidental painting”. Many modern artists use this technique as well. By pouring thin layers of two different colors atop one other, Siqueiros was able to generate seemingly spontaneous patterns like those shown above. In fact, what Siqueiros was using was a density-driven fluid instability! These patterns will only appear when a denser paint is poured atop a lighter one. They’re the result of a Rayleigh-Taylor instability – the same behavior that makes beautiful swirls of cream in coffee and the finger-like protrusions seen in supernovae.
Although a density difference is necessary to generate accidental painting, other factors like the paint layer’s thickness and viscosity affect the final pattern. For those who are mathematically-inclined, this paper has a linear stability analysis that shows how density difference, viscosity, and other factors affect the cell sizes in the pattern. (Image and research credits: S. Zetina et al.; GIF source)